Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an inherited genetic disorder most commonly associated with hemolytic anemia. Among those with G6PD deficiency, hemolytic anemia may be triggered by bacterial or viral infections and by certain foods and drugs, including the 8-aminoquinoline (8-AQ) class of antimalarials. Because 8-AQ drugs remain the only drugs approved by the U.S. Food and Drug Administration for malaria relapse prevention, the Department of Defense (DOD) requires testing of all service members' G6PD status. To estimate prevalence of G6PD deficiency among DOD service members, Composite Health Care System-generated, Health Level 7-formatted laboratory records for all service members (n=2,311,223) dated between May 2004 and Sept. 2018 were queried for G6PD testing. Corresponding demographic data were obtained from the Defense Enrollment Eligibility Reporting System. Overall prevalence of G6PD deficiency among this cohort was low, at 2.2%. Demographic trends mirrored U.S. statistics; the cohort prevalence among males (2.3%) was higher than among females (1.5%), and the prevalence among non-Hispanic blacks (9.5%) was higher than among those in any other race/ethnicity group.
What Are the New Findings?
Test results for 2,311,223 active duty service members over a 14-year period confirmed previous service-specific surveillance reports about the prevalence of G6PD deficiency. The deficiency was found in 2.2% of all service members, and the prevalence ranged from 11.2% among non-Hispanic black males to 0.3% among non-Hispanic white females. To the authors’ knowledge, this is the first DOD report widely addressing the prevalence of G6PD deficiency among active duty service members.
What Is the Impact on Readiness and Force Health Protection?
Service members who travel or deploy to malaria-endemic regions are at risk of malaria infection. Because of the risk of hemolytic anemia from use of the 8-AQ class of antimalarial drugs (e.g., primaquine, tafenoquine), military leaders should be aware of G6PD deficiency diagnoses among service members under their purview and should continue adherence to current DOD G6PD screening policies.
Background
Glucose-6-phosphate dehydrogenase (G6PD) is an essential enzyme for the pentose phosphate pathway in the erythrocyte because it converts glucose-6-phosphate to 6-phosphoglucono-d-lactone. G6PD also converts nicotinamide adenine dinucleotide phosphate (NADP) to the reduced form of NADP (NADPH), a critical redox-active cofactor that mitigates oxidative damage to the erythrocytes.G6PD deficiency is a genetic condition arising from mutations on the Gd gene on the X chromosome that encodes G6PD enzyme. As an X-linked recessive genetic disorder, G6PD deficiency has a higher prevalence in males than females.1 Reduced G6PD activity in G6PD deficient individuals can cause hemolysis with different manifestations (e.g., kernicterus in infants and hemolytic crises) from exposures to oxidative stressors including certain medications, fava beans, and infections.1,2 For example, members of the 8-aminoquinoline (8-AQ) class of antimalarial drugs can cause hemolysis in the G6PD deficient population.
8-AQ class antimalarial drugs (primaquine and the newly Food and Drug Administration (FDA)-approved tafenoquine for weekly chemoprophylaxis and single-dose radical cure) are important because they target hypnozoites—a dormant form of the malaria parasite common in Plasmodium vivax and P. ovale infections that can emerge from the liver and cause relapse days to years after treatment has cleared the malaria parasites from the circulating red blood cells. Although these infections are less often fatal than P. falciparum infections, they still can cause significant morbidity because of relapse from the dormant form of the parasites. Because the 8-AQ class drugs are the only FDA-approved drugs for malaria relapse prevention, the Department of Defense (DOD) mandated G6PD testing of service members at the time of military accession as early as 1981 (Instruction 6465.13,4 updated with DOD Instruction 6465.015 in 2015 and 6025.146 in 2018), and the Army implemented additional G6PD screening of soldiers deploying to malaria endemic regions as early as 2004.7 However, given that this drug class may trigger hemolytic anemia among those who are G6PD deficient, it is important to adhere to DOD G6PD screening instructions and provide results to command leaders and tested individuals for situational awareness and force protection.
Thus, the objectives of this surveillance report are to describe the prevalence of G6PD deficiency among active duty DOD service members who were screened during 2004–2018 and remind the DOD medical community to consider the risk of hemolytic anemia when prescribing the use of the 8-AQ class of antimalarial drugs.
Methods
This cross-sectional study used Composite Health Care System-generated, Health Level 7-formatted chemistry laboratory records from 01 May 2004 through 30 Sept. 2018 and queried for G6PD laboratory test results among all active duty, recruit, and active reserve DOD service members tested at fixed military treatment facilities (MTFs). Generally, G6PD testing occurs during accession (recruit training), which is reflected in over 90% of these records.
Key terms used to identify G6PD assays were "G6" or "glucose 6" in the test ordered or test name fields. G6PD tests are often run in panels with other genetic and hemoglobin tests. Records were excluded from the analysis if the test name field contained text indicating other genetic testing (e.g., "sickle cell anemia"), "red blood count", "hemoglobin testing", "miscellaneous". or if the test type could not be determined.
G6PD laboratory test result fields were alpha character–based or numeric. Character-based records with a test result of "Not Deficient", "High" or "Normal" were classified as "Not Deficient", Records with a test result of "Deficient" or "Low" were classified as "Deficient". Because of laboratory testing variations between MTFs, numeric test results were classified based upon the reference ranges indicated in the record. If a record had no reference range but units of measure were noted, the reference range was inferred based on other tests with the same unit of measure. In an effort to provide an accurate denominator, if the test result was numeric and the units of measure and the reference ranges could not be extrapolated from the data, the record result was classified as "Unknown." To avoid misclassification of results, if the test result field referred to the free-text result notes field, the record was classified as "Unknown".
If a service member had more than 1 record and the test results differed, the records were prioritized as follows: a "Deficient" record was retained over a "Not Deficient" or "Unknown" record, and a "Not Deficient" record was retained over an "Unknown" record. Only 1 record per service member was retained for analysis.
Final records were matched to the most recent Defense Enrollment Eligibility Reporting System (DEERS) record for each service member to obtain race/ethnicity and service affiliation. Records were not included in the final dataset if there were no matching DEERS records (n=29,642). Demographic frequencies and distributions for the final cohort were calculated using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
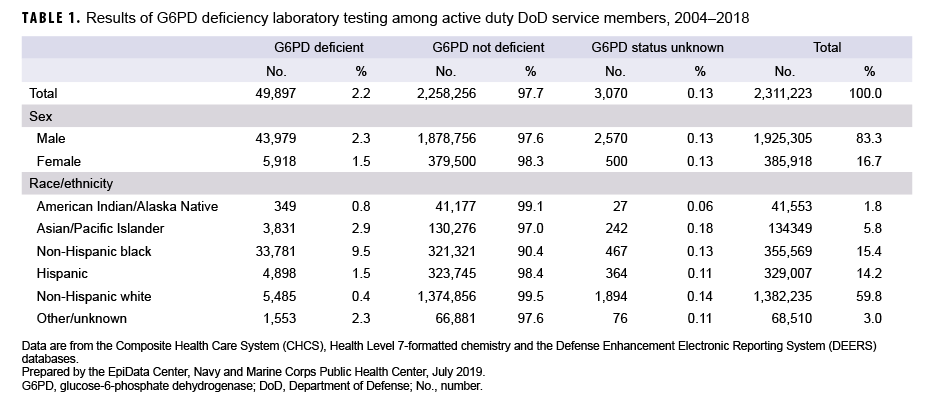
Overall results are presented in Table 1. G6PD laboratory test results for 2,311,223 service members were evaluated. Examination of laboratory records indicated that the vast majority (97.7%) of service members tested were not G6PD deficient. Only 0.13% of all records could not be classified. The majority of service members represented in this analysis (89.5%) had 1 record for G6PD testing, 8.7% had 2 records, and the remaining 1.8% had 3 or more records (data not shown). Of those with more than 1 record where the classification changed, the following changes were observed: “Not Deficient” to “Deficient” (4.6%), “Unknown” to “Deficient” (3.7%), and “Unknown” to “Not Deficient” (91.7%) (data not shown).
Of the 2,311,223 service members identified in the laboratory records as having been tested, 83.3% were male and 16.7% were female. Sex was not indicated in 3,070 records (0.13%). Males were more likely to be classified as G6PD deficient (2.3%) than females (1.5%).
Race/ethnicity was classified as “other/unknown” in 3.0% of all records (Table 1). Non-Hispanic blacks were most likely to be classified as G6PD deficient (9.5%), followed by Asians/Pacific Islanders (2.9%) and Hispanics (1.5%). Non-Hispanic whites were the least likely to be classified as G6PD deficient (0.4%).
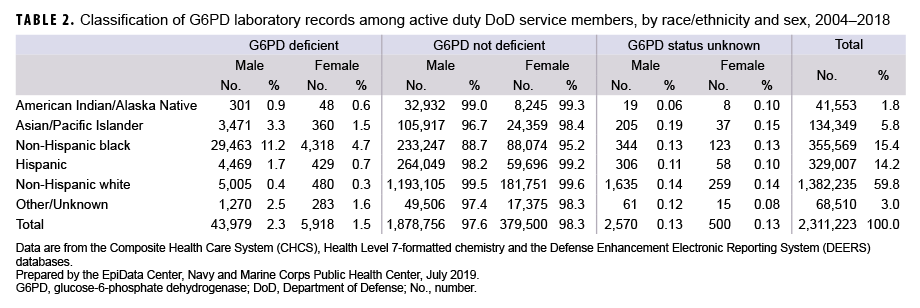
The G6PD classifications stratified by race/ethnicity and sex are shown in Table 2. Among males, non-Hispanic black service members were the most likely to be classified as G6PD deficient (11.2%), followed by Asian/Pacific Islander and Hispanic service members (3.3% and 1.7%, respectively). Non-Hispanic black males were more than twice as likely as non-Hispanic black females (4.7%) to be classified as G6PD deficient. Non-Hispanic black females had the highest proportion of G6PD deficiency of all female race/ethnicity groups. Although the non-Hispanic white race/ethnicity group represented the largest proportion of service members tested (59.8%), males and females in this race/ethnicity group were least likely to be G6PD deficient (0.4% and 0.3%, respectively).
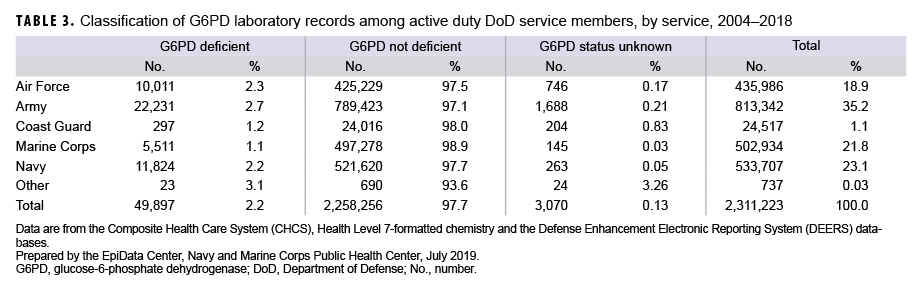
Table 3 illustrates the G6PD results by service. The proportions of service members who tested positive for G6PD deficiency were broadly similar among the services. The Army had the highest percentage of service members with G6PD deficiency (2.7%), followed by the Air Force (2.3%) and Navy (2.2%). The Coast Guard (1.2%) and the Marine Corps (1.1%) had the lowest rates of service members with G6PD deficiency.
Editorial Comment
The current study found an overall G6PD deficiency prevalence rate of 2.2% (n=49,897) among the 2,311,223 active and reserve component service members with laboratory results. To the authors’ knowledge, this is the largest study of G6PD deficiency screening among DOD service members. The results of the current analysis are consistent with other studies that have found that non-Hispanic black males are more likely to be G6PD deficient than any other race/ethnicity group.7–10
Before adoption of the newly FDA-approved tafenoquine as the primary force health protection measure for DOD service members against malaria infection, it is critical to understand the indications for prevention and treatment as well as the limitations.11 Regardless of an improved drug performance, including a radical curative efficacy, longer drug half-life, and once-weekly dosing regimen, tafenoquine still retains the risk of inducing hemolytic anemia in G6PD deficient individuals.
The results of this study must be interpreted within the context of several limitations. Laboratory records queried for this analysis were derived from fixed MTFs and do not include records from in-theater, shipboard, battalion aid station, or purchased care providers, although inclusion of these records, were they available, would not be expected to alter the findings presented herein. Genetic testing is generally performed during the accession phase of military service, so very few tests would need to be performed at austere or remote locations. Although validation steps were taken to avoid misclassification, it is possible that some results were misclassified. Because less than 1.0% of all records were classified as "Unknown", it is unlikely that these results would change the overall prevalence rates. Final laboratory records were matched to the most recent DEERS record for each service member; a service member may have changed service affiliation after the laboratory testing, but this practice is uncommon. Additionally, laboratory data were derived from DOD service members and may not be generalizable to a larger U.S. civilian population.
Given the findings of the current analysis, DOD health are providers, Combatant Commanders, and their command surgeons in areas of responsibility that include malaria-endemic regions should remain aware of the risks of 8-AQ hemolytic anemia in G6PD deficient service members. In addition, targeted health education and risk management instruction should be provided to G6PD deficient service members in order to mitigate adverse health outcomes. Furthermore, continued implementation of the DOD's G6PD screening instruction is required to mitigate G6PD deficiency-associated adverse events.
Author affiliations: Malaria Vaccine Branch, Walter Reed Army Institute of Research, Silver Spring, MD (MAJ Jangwoo Lee); Defense Health Agency, Combat Support, Health Care Operations, Public Health Division, Armed Forces Health Surveillance Branch, Navy Satellite (Beth T. Poitras)
Acknowledgments: The authors thank LTC Chad C. Black, LTC Mara Kreishman-Deitrick, COL James E. Moon, COL Viseth Ngauy, MAJ Brandon S. Pybus, and Angela D. Schlein for their support of this analysis and report.
Disclaimer: The views expressed in this report are those of the authors and do not necessarily reflect the official policy or position of the Armed Forces Health Surveillance Branch, the Defense Health Agency, the Department of the Army, the Department of the Navy, the Department of Defense, or the U.S. Government. Analytic support was provided by the Armed Forces Health Surveillance Branch of the Public Health Division at the Defense Health Agency. Authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C., §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- Richardson SR, O'Malley GF. (2019). Glucose 6 phosphate dehydrogenase (G6PD) deficiency. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK470315/. Accessed 23 Jan. 2019.
- Luzzatto L, Arese P. Favism and glucose-6-phosphate dehydrogenase deficiency. N Eng J Med. 2018;378(1):60–71.
- Department of Defense Instruction 6465.1. Hemoglobin S and erythrocyte glucose-6-phosphate dehydrogenase deficiency test program. 29 July 1981.
- Webber BJ, Witkop CT. Screening for sickle-cell trait at accession to the United States military. Mil Med. 2014;179(11):1184–1189.
- Department of Defense Instruction 6465.01. Erythrocyte glucose-6-phosphate dehydrogenase deficiency (G6PD) and sickle cell trait screening programs. 17 July 2015.
- Department of Defense Instruction 6025.14. Active duty service members (ADSM) erythrocyte glucose-6-phosphate dehydrogenase (G6PD) deficiency and sickle cell trait (SCT) screening. 6 Dec. 2018.
- Chinevere TD, Murray CK, Grant E Jr, Johnson GA, Duelm F, Hospenthal DR. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil Med. 2006;171(9):905–907.
- Heller P, Best WR, Nelson RB, Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. N Eng J Med. 1979;300(18):1001–5.
- Tarlov AR, Brewer GJ, Carson PE, Alving AS. Primaquine sensitivity. Glucose-6-phosphate dehydrogenase deficiency: an inborn error of metabolism of medical and biological significance. Arch Intern Med. 1962;109:209–234.
- Uddin DE, Dickson LG, Brodine CE. Glucose-6-phosphate dehydrogenase deficiency in military recruits. JAMA. 1974;227(12):1408–1409.
- Fukuda M, Wojnarski M, Martin N, Zottig V, Waters NC. Editorial: Malaria in the Korean Peninsula: risk factors, latent infections, and the possible role of tafenoquine, a new antimalarial weapon. MSMR. 2018;25(11):2–3.