Abstract
This report uses ICD-9 and ICD-10 codes (277.7 and E88.81, respectively) for the metabolic syndrome (MetS) to summarize trends in the incidence and prevalence of this condition among active component members of the U.S. Armed Forces between 2002 and 2017. During this period, the crude overall incidence rate of MetS was 7.5 cases per 100,000 person-years (p-yrs). Compared to their respective counterparts, overall incidence rates were highest among Asian/Pacific Islanders, Air Force members, and warrant officers and were lowest among those of other/unknown race/ethnicity, Marine Corps members, and junior enlisted personnel and officers. During 2002–2017, the annual incidence rates of MetS peaked in 2009 at 11.6 cases per 100,000 p-yrs and decreased to 5.9 cases per 100,000 p-yrs in 2017. Annual prevalence rates of MetS increased steadily during the first 11 years of the surveillance period reaching a high of 38.9 per 100,000 active component service members in 2012, after which rates declined slightly to31.6 per 100,000 active component service members in 2017. Validation of ICD-9/ICD-10 diagnostic codes for MetS using the National Cholesterol Education Program Adult Treatment Panel III criteria is needed to establish the level of agreement between the two methods for identifying this condition.
What Are the New Findings?
This analysis confirmed that the incidence of metabolic syndrome (MetS) among service members steadily increases with advancing age. However, because the specific MetS ICD codes tend to be underutilized in patient records, this analysis' estimates of incidence greatly underestimate rates that have been derived using biologic thresholds for the five components of the syndrome.
What Is the Impact on Readiness and Force Health Protection?
MetS is a medically disqualifying condition for appointment, enlistment, or induction into military service. Significant impacts on force readiness include administrative discharges for failure to meet weight standards, non-deployability due to type 2 diabetes diagnosis requiring medication, and increased potential for cardiovascular disease in deployed environments.
Background
The metabolic syndrome (MetS) is a cluster of cardiometabolic risk factors that is associated with increased risk of multiple chronic diseases and premature mortality.1,2 MetS is characterized by abdominal obesity, dyslipidemia (elevated plasma triglycerides and/or reduced high density lipoprotein [HDL] cholesterol), elevated fasting plasma glucose level, and hypertension.2 The association between MetS and increased risk of multiple chronic diseases (e.g., cardiovascular disease, chronic liver disease, chronic kidney disease, arthritis, and several types of cancer) and all-cause mortality is well established.3–10 However, there is some uncertainty regarding whether MetS confers risk over and above its individual components.11,12 Regardless of whether MetS is considered to have unique predictive value, the importance of identifying and managing its individual components to decrease morbidity and mortality associated with diabetes and cardiovascular disease is undisputed.2,11,13
The importance of MetS was highlighted in 2001 with the approval of the ICD-9 code, 277.7, for “dysmetabolic syndrome X”.14 However, studies using data from the National Hospital Discharge Survey and the National Ambulatory Medical Care Survey as well as large U.S. civilian administrative databases have found that a diagnosis of MetS is rarely recorded in these data using the designated ICD code.15–18 Given this under-recording, most efforts to better estimate the public health burden of MetS in the civilian population have relied on biologic thresholds of five components of MetS (elevated waist circumference, elevated triglyceride level, reduced HDL cholesterol level, elevated blood pressure, and elevated fasting blood glucose level). These efforts have been complicated by the lack of consistency in the clinical parameters used to define MetS; however, the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII) criteria are one of the most widely agreed upon criteria for MetS.19 These criteria do not include the designated ICD code for MetS but define MetS by the presence of at least three of the five components based on laboratory and vital data. Studies using this definition and data from the National Health and Nutrition Examination Survey (NHANES) estimated the overall age-adjusted prevalence of MetS in 1988–1994 to be 25.3%.20 In 1999–2006, the overall age-adjusted prevalence declined slightly to 25.0%20 and then increased to 34.3% in 2007–2014.21 Examination of trends from 2007 through 2014 showed that the prevalence of MetS remained relatively stable in all age, sex, and race/ethnicity groups.21
The majority of studies that have examined the prevalence of MetS among U.S. military personnel have done so among specific subpopulations (e.g., soldiers at accession, veterans with or without cardiovascular disease or diabetes mellitus, those exposed to environmental hazards).22–34 However, few studies have examined the prevalence of MetS among active component service members.34 Using electronic health record data from the Military Health System (MHS) Management Analysis and Reporting Tool and the NCEP-ATPIII criteria for defining MetS, Herzog et al. demonstrated a trend of decreasing prevalence of MetS among a sample of active duty service members during fiscal years 2009–2012.34 Age-adjusted MetS prevalence among male service members in the sample decreased from 24.7% in 2009 to 21.1% in 2012. The decrease in age-adjusted prevalence was not as pronounced among female service members (10.0% in 2009 to 8.3% in 2012).34
To determine and document the long term trends in diagnoses of MetS in the active component population, the current report summarizes trends in the incidence and prevalence of MetS using the ICD-9 and ICD-10 codes for this condition among active component members of the U.S. Armed Forces during 2002–2017.
Methods
The surveillance period was Jan. 1, 2002 through Dec. 31, 2017. The surveillance population consisted of all individuals who served in the active component of the Army, Navy, Air Force, or Marine Corps at any time during the surveillance period. Cases of MetS were identified by ICD-9 diagnostic code 277.7 and ICD-10 code E88.81 recorded in standardized records of inpatient and outpatient encounters in military and non-military medical facilities documented in the Defense Medical Surveillance System (DMSS).
An incident case of MetS was defined by hospitalization with a case-defining diagnostic code in any diagnostic position or by two or more outpatient diagnoses between 1 and 180 days apart, with at least one of these diagnoses in a primary diagnostic position. An individual could be counted as an incident case of MetS once per lifetime. Individuals who met the case-definition for MetS prior to the surveillance period (i.e., prevalent cases) were excluded from the incidence rate calculation. Incidence rates were calculated as incident MetS diagnoses per 100,000 person years (p-yrs) of active component service. Both deployed and non-deployed person time were used in the denominator.
Lifetime prevalence of the diagnoses of MetS was estimated for each year in the 16-year surveillance period. The numerator for annual lifetime prevalence calculations consisted of service members who had ever been diagnosed with MetS and who were in service during the given calendar year. The denominator for annual prevalence calculations consisted of the total number of active component service members who served at any time during the given year. Annual prevalence estimates were calculated as the number of prevalent cases per 100,000 active component service members.
Results
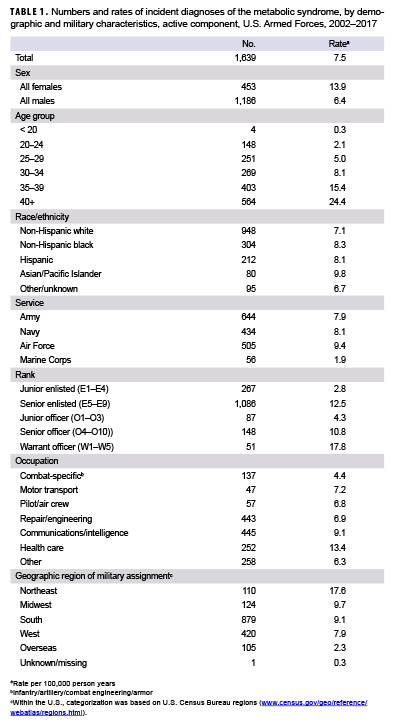
During 2002–2017, a total of 1,639 active component service members received incident diagnoses of MetS, for a crude overall incidence rate of 7.5 cases per 100,000 p-yrs (Table 1). The overall incidence of MetS among females was more than twice that of males (13.9 per 100,000 p-yrs and 6.4 per 100,000 p-yrs, respectively). Crude overall rates of MetS increased with increasing age; the greatest percent increase in overall incidence rates occurred between those aged 30–34 years and those aged 35–39 years or older (Table 1). Across all cases, the median age at the time of incident MetS diagnosis was 36 years (interquartile range [IQR]=30–41) with females having a much younger median age at diagnosis (31 years; IQR=26–38) compared to males (38 years; IQR=32–42) (data not shown).
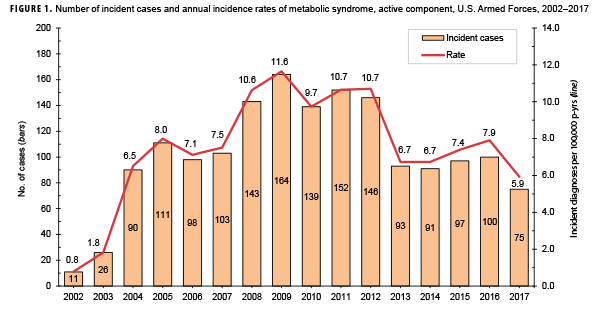
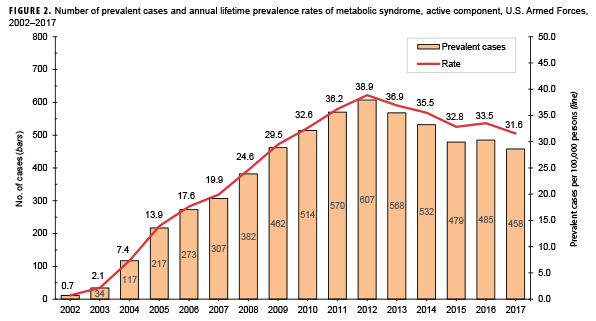
Compared to their respective counterparts, overall incidence rates were highest among Asian/Pacific Islanders (9.8 per 100,000 p-years), Air Force members (9.4 per 100,000 p-yrs), and warrant officers (17.8 per 100,000 p-yrs) and lowest among those of other/unknown race/ethnicity (6.7 per 100,000 p-yrs), Marine Corps members (1.9 per 100,000 p-yrs), and junior enlisted personnel and officers (2.8 and 4.3 per 100,000 p-yrs, respectively). Stratification by military occupation revealed that crude overall rates of MetS were highest among healthc are workers (13.4 per 100,000 p-yrs) and lowest among those in combat-specific occupations (4.4 per 100,000 p-yrs) (Table 1). Of active component service members with known locations of military assignment, overall incidence of MetS was highest among those stationed in the Northeastern region of the U.S. (17.6 per 100,000 p-yrs) and lowest among those stationed overseas (2.3 per 100,000 p-yrs) or in the West (7.9 per 100,000 p-yrs). During 2002–2017, the crude annual incidence rates of MetS peaked in 2009 at 11.6 cases per 100,000 p-yrs and decreased to 5.9 cases per 100,000 p-yrs in 2017 (Figure 1). Crude annual prevalence rates of MetS increased steadily during the first 11 years of the surveillance period reaching a high of 38.9 per 100,000 active component service members in 2012, after which rates declined slightly to 31.6 per 100,000 active component service members in 2017 (Figure 2).
Editorial Comment
The results of the current study show a trend of steadily increasing prevalence of MetS diagnoses (based on ICD-9/ICD10 codes alone) among active component service members between 2002 and 2012 followed by a slight decline during the subsequent five years. In contrast, the findings of Herzog et al. demonstrated a decrease in MetS prevalence in a sample of active duty service members between 2009 and 2012,34 while the MetS prevalence estimates for the general U.S. population (NHANES) remained relatively stable during 2009–2014.21 However, because the estimates from the Herzog et al. and U.S. general population-based studies used different sets of modified NCEP-ATP III criteria to identify MetS cases, they are not directly comparable to those obtained in the current analysis which used only ICD-9/ICD-10 diagnostic codes. The most comparable prevalence estimate available at the time of this report was obtained from a recent study of cardiometabolic risk factors among soldiers who accessed into the U.S. Army during 2001–2011. Using the ICD-9 diagnostic code to identify MetS cases, Hruby et al. obtained a prevalence estimate of 31.2 MetS diagnoses per 100,000 Army entrants during the 11-year period.17
Despite the major differences in case definitions used in studies of the prevalence of MetS, several broad findings of the current analysis are consistent with the literature. The age-related rise in the prevalence of MetS is well established and has been attributed to the development of central obesity in middle age associated with overeating and a decline in physical activity.35,36 In contrast to the results of general population-based studies using NHANES data and the findings of Herzog and colleagues' study of MetS prevalence among a sample of active duty service members, overall rates of MetS in the current study were higher in women than in men.21,34,37 The reasons for this difference are unclear but are likely related to sex differences in the prevalence of individual MetS components in the study population.37 The pattern of lower incidence rates observed among junior enlisted and junior officers compared to those in other grades is likely highly correlated and confounded by age. In addition, the finding that overall incidence rates of MetS were lowest among Marine Corps members may be related to differences in the age and obesity distributions of the services.38,39 The decline in the prevalence of MetS during the surveillance period might be due to earlier identification of MetS and/or more aggressive management of its component conditions. Data on trends in incidence of MetS in the general U.S. population using ICD diagnostic codes (either alone or in conjunction with other diagnostic codes and/or laboratory data relevant to components of MetS) during a comparable time period were not available at the time of this report, thus precluding comparisons to the current results. This lack of comparable results is likely due, at least in part, to the under-recording of MetS using the designated ICD code in U.S. civilian administrative databases.
Whether the designated ICD code for MetS is underutilized in the MHS is unknown. However, several potential reasons for the low coding rate of MetS in the civilian health system have been posited. Some health care providers may think that the MetS is not a meaningful concept and thus lacks clinical utility.11,40 In addition, it is possible that lack of or limited reimbursement for MetS by some commercial insurers may lead health care providers to focus on using diagnostic codes that can receive reimbursement (e.g., diabetes, hypertension).41 Low frequency of coding also may be due to concern regarding the impact of the use of the MetS diagnostic code on life insurance policies and health insurance portability.42 Additionally, limited coding time during clinic appointments may be devoted to other more urgent clinical conditions.43,44
It is important to note that, because diagnostic codes from inpatient and outpatient medical records were used as proxies for incident and prevalent cases, the validity of the current results depends upon the accuracy of a physician-assigned diagnosis of MetS. Furthermore, the pattern of, and trends in, prevalence and incidence rates reported here may be due, to an unknown degree, to variations in coding practices. Validation of ICD-9/ICD-10 diagnostic codes for MetS using ATP III criteria modified for the MHS electronic health record data available (e.g., self-reported height and weight) is needed to establish the level of agreement between the two methods for identifying this condition.
Another limitation of the current analysis is related to the implementation of MHS GENESIS, the new electronic health record for the Military Health System. For 2017, medical data from sites that were using MHS GENESIS are not available in DMSS. These sites include Naval Hospital Oak Harbor, Naval Hospital Bremerton, Air Force Medical Services Fairchild, and Madigan Army Medical Center. Therefore, medical encounter and person-time data for individuals seeking care at one of these facilities during 2017 were not included in the analysis.
MetS has obvious effects on accession into and retention in the military, most notably with respect to maintenance of flight status or the requirements of other special missions. Additional significant impacts of MetS on force readiness are based on administrative discharges for failure to meet weight standards, non-deployability due to type 2 diabetes diagnosis requiring medication, and increased potential for cardiovascular disease in deployed environments.45-47 Future investigations of the incidence and prevalence of MetS among active component service members should employ a fuller range of available administrative data including the diagnostic codes for the individual components of MetS, codes pertaining to the biologic thresholds for these components, and documentation of prescription medication used to treat these component conditions.
Acknowledgments: The authors thank COL Babette C. Glister (Walter Reed National Military Medical Center, Bethesda, MD) for her review of this work and valuable input into its content.
References
- Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17): 2735–2752.
- Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–1778.
- Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25(6):375–384.
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 56(14):1113–1132.
- Bjørge T, Lukanova A, Jonsson H, Tretli S, Ulmer H, Manjer J, et al. Metabolic syndrome and breast cancer in the Me-Can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1737–1745.
- Borena W, Strohmaier S, Lukanova A, Bjørge T, Lindkvist B, Hallmans G, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131(1):193–200.
- Borena W, Edlinger M, Bjørge T, Häggström C, Lindkvist B, Nagel G, et al. A prospective study on metabolic risk factors and gallbladder cancer in the metabolic syndrome and cancer (Me-Can) collaborative study. PLoS One. 2014;9(2):e89368.
- Lindkvist B, Johansen D, Stocks T, Concin H, Bjørge T, Almquist M, et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer. 2014;14(1):103.
- Stocks T, Bjørge T, Ulmer H, Manjer J, Häggström C, Nagel G, et al. Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol. 2015;44(4):1353–63.
- Gale E. Should we dump the metabolic syndrome? Yes. BMJ. 2008;336(7645):640.
- López-Suárez A, Bascuñana-Quirell A, Beltrán-Robles M, Elvira-González J, Fernández-Palacín F, Barroso-Casamitjana E, SolinoOcaña. Metabolic syndrome does not improve the prediction of 5-year cardiovascular disease and total mortality over standard risk markers. Prospective population based study. Medicine (Baltimore). 2014;93(27):e212.
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2009;23:469–480.
- National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Atlanta, GA: NCHS; 2002.
- Ford ES. Rarer than a bluemoon: the use of a diagnostic code for the metabolic syndrome in the US. Diab Care. 2005;28:1808–1809.
- Birnbaum HG, Mattson ME, Kashima S, Williamson TE. Prevalence rates and costs of metabolic syndrome and associated risk factors using employee’s integrated laboratory data and health care claims. J Occup Environ Med. 2011;53(1):27– 33.
- Goetzel R, Kent K, Henke RM, Pack C, D’Arco M, Thomas J, et al. Prevalence of metabolic syndrome in an employed population as determined by analysis of three data sources. J Occup Environ Med. 2015;59(5):e103–e104.
- Motiwala T, Kite B, Regan K, Gascon GM, Payne P. Domain analysis of integrated data to reduce cost associated with liver disease. Stud Health Technol Inform. 2015;216:414–418.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645.
- Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1998–2012. Prev Chronic Dis. 2017;14:E24.
- Shin D, Kongpakpaisarn K, Bohra C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int J Cardiol. 2018;259:216–219.
- Hruby A, Bulathsinhala L, McKinnon CJ, Hill OT, Montain SJ, Young AJ, et al. Body mass Iidex at accession and incident cardiometabolic risk factors in U.S. Army soldiers, 2001–2011. PLoS One. 2017;12(1):e0170144.
- Linnville S, Hoyt RE, Moore JL, Segovia F, Hain RE. Posttraumatic stress disorder and metabolic syndrome: retrospective study of repatriated prisoners of war. Mil Med. 2011;176(4):369–374.
- Khatana SA, Kane J, Taveira TH, Bauer MS, Wu WC. Monitoring and prevalence rates of metabolic syndrome in military veterans with serious mental illness. PLoS One. 2011;6(4): e19298.
- Phillips AC, Carroll D, Gale CR, Drayson M, Thomas GN, Batty GD: Lymphocyte subpopulation cell counts are associated with the metabolic syndrome and its components in the Vietnam Experience Study. Atherosclerosis. 2010;213(1):294–298.
-
- Batty GD, Gale CR, Mortensen LH, Langenberg C, Shipley MJ, Deary IJ. Pre-morbid intelligence, the metabolic syndrome and mortality: the Vietnam Experience Study. Diabetologia. 2008;51(3):436–443.
- Krasuski RA, Devendra GP, Cater G, Whitney EJ. The effect of gemfibrozil, niacin and cholestyramine combination therapy on metabolic syndrome in the Armed Forces Regression Study. Am J Med Sci. 2011;341(5):378–382.
- Blanchard MS, Eisen SA, Alpern R, et al: Chronic multisymptom illness complex in Gulf War I veterans 10 years later. Am J Epidemiol. 2006;163(1):66–75.
- Radjen SD, Jovelic AS, Radjen GS, Hajdukovic ZV, Radakovic SS: Metabolic syndrome and carotid artery intima-media thickness in military pilots. Aviat Space Environ Med. 2011; 82(6):622–626.
- Zhao XQ, Krasuski RA, Baer J, Albers JJ, Brown BG: Effects of combination lipid therapy on coronary stenosis progression and clinical cardiovascular events in coronary disease patients with metabolic syndrome: a combined analysis of the Familial Atherosclerosis Treatment Study (FATS), the HDL-Atherosclerosis Treatment Study (HATS), and the Armed Forces Regression Study (AFREGS). Am J Cardiol. 2009;104(11):1457–1464.
- Gupta A, Gupta S, Pavuk M, Roehrborn CG: Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Urology. 2006;68(6):1198–11205.
- Clearfield M, Downs JR, Lee M, Langendorfer A, McConathy W, Gotto AM Jr: Implications from the Air Force/Texas Coronary Atherosclerosis Prevention Study for the Adult Treatment Panel III guidelines. Am J Cardiol. 2005;96(12):1674–1680.
- Cranston MM, True MW, Wardian JL, Carriere RM, Sauerwein TJ. When military fitness standards no longer apply: the high prevalence of metabolic syndrome in recent Air Force retirees. Mil Med. 2017;182(7):e1780–e1786.
- Herzog CM, Chao SY, Eilerman PA, Luce BK, Carnahan DH. Metabolic syndrome in the Military Health System based on electronic health data, 2009–2012. Mil Med. 2015;180(1):83–90.
- Sumner AD, Sardi GL, Reed JF. Components of the metabolic syndrome differ between young and old adults in the US population. J Clin Hypertens. 2012;14:502–6.
- Devers MC, Campbell S, Simmons D. Influence of age on the prevalence and components of the metabolic syndrome and the association with cardiovascular disease. BMJ Open Diabetes Res Care. 2016;4(1):e000195.
- Beltrán-Sánchez H, Harhat MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62(8)697–703.
- Department of Defense, Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy (ODASD (MC&FP)). (2017). 2016 Demographics: Profile of the Military Community. Washington, DC.
- Department of Defense. DOD Health Related Behaviors Survey of Active-Duty Service Members: Final Report. (2015). Santa Monica, CA: RAND Corporation.
- Preiss D, Sattar N. Metabolic syndrome: collapsing under its own weight? Diabetic Med. 2009;26(5):457–459.
- Reynolds K, Muntner P, Fonseca V. Metabolic syndrome underrated or underdiagnosed? Diabetes Care. 2005;28:1831–1832.
- Medscape. CME/CE. The obesity epidemic: Prevention and treatment of the metabolic syndrome. Diagnosing metabolic syndrome. https://www.medscape.org/viewarticle/441282_4. Accessed on 21 Sept. 2018.
- Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of ICD9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424-1241.
- Martin B-J, Chen G, Graham M, Quan H. Coding of obesity in administrative hospital discharge abstract data: accuracy and impact for future research studies. BMC Health Serv Res. 2014;14(1):70.
- Department of Defense. Instruction 6490.07, Deployment-Limiting Medical Conditions for Service Members and DOD Civilian Employees. Feb. 2010. http://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/649007p. pdf. Accessed on 30 Nov. 2018.
- U.S. Department of the Army. Army Regulation 40–501: Standards of Medical Fitness, 14 June 2017. U.S. Department of the Army, Washington, D.C. https://armypubs.army. mil/ProductMaps/PubForm/Details.aspx?PUB_ ID=1002549. Accessed on 26 Nov. 2018.
- Department of Defense. Instruction 6130.03, Medical Standards for Appointment, Enlistment, or Induction into the Military Services. Change 1. 30 March 2018. http://www.esd. whs.mil/Portals/54/Documents/DD/issuances/ dodi/613003p.pdf. Accessed on 6 Oct. 2018.