Abstract
Hepatitis C virus (HCV) infection rates are rising in the U.S. despite widely available tools to identify and effectively treat nearly all of these cases. This cross-sectional study aimed to use laboratory data to evaluate the prevalence of HCV diagnoses among active component U.S. military service members, describe the characteristics of those diagnosed with HCV, and evaluate the adherence of their care to current standards of practice. All service members in the active component U.S. military between Jan. 1 and Dec. 31, 2020 were included in the study population. The primary outcome was an HCV diagnosis at any time during military service, with secondary outcomes of HCV treatment and sustained virologic response (SVR). The initial case-finding algorithm used laboratory data to identify HCV patients seen in infectious disease and gastrointestinal disease clinics in military treatment facilities (MTFs) (direct care); this was supplemented with additional data to assess and correct for undercounting from cases occurring outside MTFs (purchased care). Thirty active component service members in 2020 had been diagnosed with HCV infection during their military service via direct care, or an estimate of 68 cases after correcting for additional cases from purchased care; this number represents only 12% of the expected number of infections based on previous studies. Of the 30 cases treated via direct care, 28 (93%) received HCV treatment, with 27 of those 28 (96%) achieving SVR. Changes to HCV screening policy for military accessions should be considered in order to effectively identify and treat asymptomatic HCV infections that would otherwise go undetected.
What Are the New Findings?
Among active component service members with a diagnosis of HCV during military service at MTFs, 93% received appropriate treatment, and 96% of those treated had a documented sustained viral response. However, this study also suggests that only 12% of the expected HCV infections in this population were diagnosed.
What Is the Impact on Readiness and Force Health Protection?
Although HCV infection results in a low risk of progression to symptomatic disease during military service, it poses a risk to operational requirements such as the walking blood bank. Recent changes in HCV epidemiology and national guidelines, as well the impact on veterans' health, suggest that HCV screening policies, particularly at accession, should be reevaluated.
Background
During 2013–2016, an estimated 4.1 million U.S. adults were hepatitis C virus (HCV) antibody positive indicating either past or current infection with HCV, while 2.4 million had an active infection based on a positive HCV RNA test.1 Due to the introduction of novel direct acting antiviral medications (DAAs) in the early 2010s, greater than 90% of cases of chronic HCV infection can be cured prior to progression to advanced liver disease.2 Despite these recent advancements in treatment, only 49% of those with commercial insurance who are aware of their diagnosis of chronic HCV infection receive treatment,3 and HCV remains the leading cause of cirrhosis in North America and the second leading cause worldwide.4
To better target interventions which promote the control of HCV and monitor its progress, the World Health Organization has developed a consensus HCV cascade of care.5 This cascade of care depicts how many of those infected with HCV have progressed through the sequence of stages required for effective HCV control, including diagnosis, treatment, and cure. The goal of the cascade of care is to identify the stages at which the greatest numbers of infected individuals are lost to care, in order to target control efforts most effectively and efficiently towards these stages.
It is of concern to the military that HCV rates are increasing in younger, military-aged Americans, largely due to the ongoing opioid epidemic in the U.S.6,7 Since most chronic HCV infections are asymptomatic, persons infected are often not aware of their underlying condition until it has progressed to advanced liver disease. Because of these factors, as well as the low level of adoption of previous screening recommendations, the Centers for Disease Control (CDC) and U.S. Preventive Services Task Force (USPSTF) recently implemented updated screening policies in 2020 recommending that all persons aged 18 to 79 undergo a one-time screening for HCV infection.8,9
Within the U.S. military, HCV infection not only poses risks to health and readiness of those already infected, it also poses a risk of transmission to previously uninfected service members when utilizing the walking blood bank where whole blood transfusions are given during emergency situations in combat.10 A prior investigation into the prevalence of chronic HCV infection in a random serum sample of the deployed population between 2007 and 2010 reported a rate of 43 per 100,000 service members.11 Knowledge of the current epidemiology of HCV infection within the active component U.S. military population is important in informing the Department of Defense (DOD) policies for the screening, diagnosis, and treatment of HCV.
Per current DOD guidelines, applicants for entry into military service are medically disqualified if they display a "history of chronic hepatitis C, unless successfully treated and with documentation of a cure 12 weeks after completion of a full course of therapy."12 All applicants are required to submit a medical history prior to accession, and each branch has its own system of screening. In 2012, the Navy and Marine Corps updated their accession screening standards to require all new applicants to undergo HCV screening prior to entering military service.13 The Army and Air Force currently do not require HCV testing at accession.
The objective of this study was to use laboratory surveillance data from the population of U.S. active component service members in 2020 to estimate the period prevalence of HCV diagnosis at any point in time during their military service, to assess associated risk factors for HCV diagnosis, and assess the cascade of HCV care.
Methods
This cross-sectional study examined U.S. military service members in the active component between Jan. 1 and Dec. 31, 2020. The primary outcome was prevalent HCV diagnosis at any time during military service, with secondary outcomes of HCV treatment and viral suppression. Potential cases were initially identified by the Navy and Marine Corps Public Health Center (NMCPHC) through active laboratory-based case finding from the active component military population from Jan. 1, 2011 to Dec. 31, 2020 using the Composite Health Care System (CHCS) health level 7 (HL7) laboratory databases. This laboratory-based case-finding algorithm identified positive HCV laboratory results from HCV antibody, RNA, or genotyping tests ordered through Gastroenterology (GI) or Infectious Disease (ID) clinics at fixed military treatment facilities (MTFs). CHCS HL7 data do not include records from shipboard facilities, battalion aid stations, purchased care, or in-theater facilities. Although most of these cases were newly diagnosed, it is likely that many existed prior to accession into military service; given this, it is more accurate to call this a prevalence rather than an incidence study. Purchased care was defined as all care provided outside of (MTFs) by non-military providers but paid for by the military health system. Demographic data and dates of military service were obtained from the Armed Forces Health Surveillance Division's Defense Medical Surveillance System (DMSS). All potential cases identified by laboratory surveillance were verified by chart review using the Armed Forces Health Longitudinal Technology Application (AHLTA) military electronic medical record.
Potential cases met the study case definition for HCV diagnosis if they had a positive HCV RNA laboratory result at any point during their military service, although as mentioned above, laboratory data were only available between 2011 and 2020. Individuals were excluded if they ended military service prior to Jan. 1, 2020. Chart review was systematically performed on all potential cases by 1 of the authors (ML) in June 2021 after an initial validation of 10% of the cases by another of the authors (JM). Chart review consisted of extraction of laboratory data (including HCV RNA, antibody, and genotyping dates and results), medication information (type, dates, and number prescribed), and clinical data (provider assessment of testing indication and source of infection).
After completing the analysis using the original case-finding algorithm, there were residual concerns that this algorithm had only identified those who had received care in MTFs (direct care), which may have resulted in undercounting cases if they had been diagnosed or treated outside MTFs (purchased care). To assess the magnitude of this undercounting, an additional data query and analysis was performed, this time without restricting to those labs ordered by military GI or ID clinics. To accommodate computing and personnel limitations in data acquisition capabilities, this analysis was restricted to cases initially diagnosed during calendar year 2019 only. A second set of chart reviews of the electronic medical records was performed for all individuals identified in this data query, and these results were used to compare with and correct the results from the original case-finding algorithm.
The size of the population at risk was obtained from the 2019 Annual Demographics Profile published by Military One Source.14 Prevalence was calculated as prevalent cases of HCV per 100,000 active component service members and prevalence ratios (PRs) were used to assess associations with demographic characteristics. The cascade of care for HCV included HCV diagnosis, receipt of HCV treatment with DAAs, and achievement of SVR after treatment.5 Measures of frequency were used in the cascade of care analysis. Year of diagnosis was used to assess the impact of the Navy and Marine Corps screening policy implemented in late 2012. To accomplish this, the diagnosis of cases during the period of 2013 to 2020 was compared to the period 2003 to 2012. Stata version 15 (2017, Statacorp LP, College Station, TX) was used for all statistical analysis.
Results
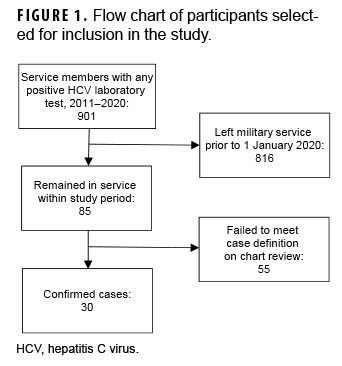
The initial laboratory surveillance data provided by NMCPHC identified 901 potential cases with positive HCV-related tests between 2011 and 2021. However, 816 of those identified had left military service prior to 2020 (Figure 1). After detailed chart review on the remaining 85 subjects, only 30 met the case definition for HCV diagnosis, which included a positive test for HCV RNA. All 30 had 1 or more provider diagnoses of HCV in their medical records. Of the 55 excluded subjects who failed to meet the case definition after chart review, 21 had cured HCV infection as evidenced by a positive HCV antibody test but a negative HCV RNA test result, 1 had a positive HCV antibody but no history of RNA confirmatory testing, 13 had hepatitis B infection, 1 had hepatitis E, and 19 had other hepatic conditions.
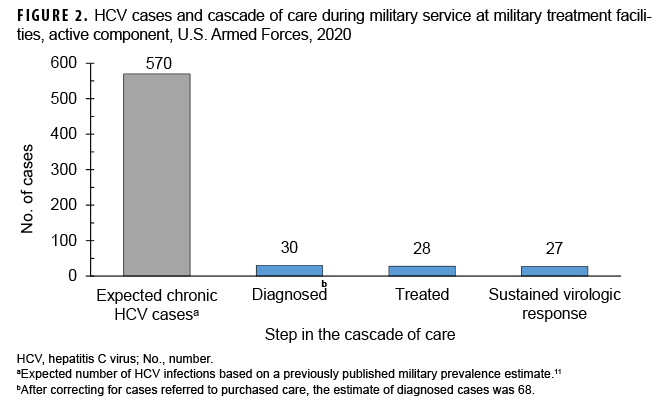
The period prevalence of HCV diagnosis occurring during military service at MTFs was 2.3 per 100,000 service members (Table), representing 5.3% of the expected infections (n=570) based on previously estimated prevalence.11 Diagnosed cases were predominantly male service members and the majority of cases were in the Army. Diagnosed cases showed a bimodal distribution by age with the highest prevalence among those aged 41 or older (5.8 per 100,000) and those aged 26–30 (4.3 per 100,000). Cases were predominantly non-Hispanic White or non-Hispanic Black service members with very few cases among persons from other race/ethnicity groups.
The additional data query for 2019 that included all clinics (not restricted to GI or ID), which was performed to examine completeness of the original case-finding algorithm, revealed 51 unique individuals with positive HCV tests. Of these 51, 14 had already been identified by the original algorithm, 11 who were counted in 2019 and 3 in previous years. Of the remaining 37 individuals which had not been identified previously, 5 were HCV antibody positive but PCR negative, 3 had been diagnosed prior to 2019, and 2 were administrative errors. Of the remaining 27 potential cases, 13 left or were in the process of leaving military service prior to Jan. 1, 2020, which was a criterion for exclusion from the study. The 14 remaining individuals were confirmed HCV cases but had been referred to purchased care, which is why they were not identified by the original algorithm. This suggests that only 44% (11/25) of HCV cases in the active component military are treated in the direct care system and that the original algorithm resulted in undercounting by 56%. After correcting for this undercounting, a revised estimate of 68 cases (12% of expected) was generated, a period prevalence of 5.2 per 100,000. Of note, all 14 of the additional cases missed by the original algorithm were diagnosed and cared for by civilian ID or GI providers, and all 14 also had evidence of treatment in the electronic medical record. It was more difficult to ascertain whether patients referred to purchased care had been cured, as PCR results were sometimes not available after treatment. Nevertheless, 11 of these patients had direct evidence of cure in the medical record, and the other 3 had statements by treating physicians which suggested (but did not confirm) that cure had been achieved.
Of the 30 HCV cases diagnosed in direct care, 28 (93%) received appropriate medical therapy (Figure 2). The 2 untreated cases were offered therapy but elected to defer treatment because of personal considerations. Of note, 1 case who spontaneously cleared without treatment was considered as treated in this analysis. Of the 28 treated, 27 (96%) had confirmed viral suppression on follow-up testing, with 1 case failing to follow up for confirmation of viral suppression after treatment.
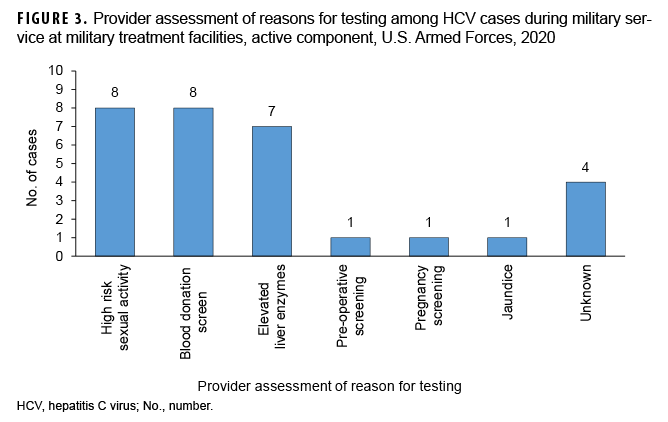
Providers documented that nearly all of the HCV diagnoses were discovered by screening of asymptomatic patients (Figure 3). The only symptomatic HCV case was a patient who presented with jaundice.
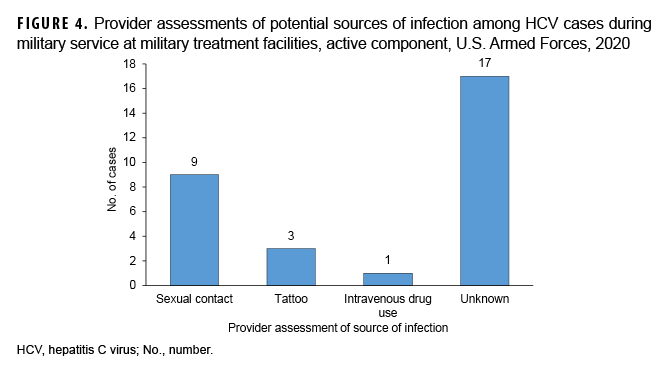
Provider assessment of potential sources of infection showed the majority of cases (n=17; 57%) did not identify a potential source of infection; 30% (n=9) were attributed to sexual contact with an infected individual; and only 3% (n=1) of cases were attributed to intravenous drug use (Figure 4).
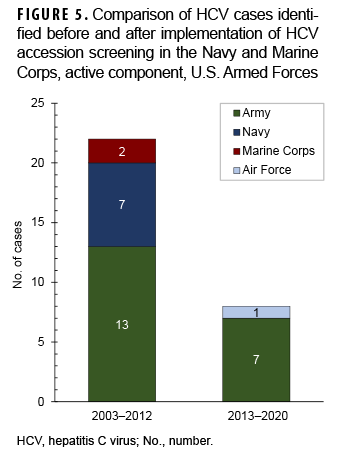
None of the cases identified since the 2013 implementation of the accession screening program in the Navy and Marine Corps occurred in either of these branches (Figure 5). All cases of HCV identified after 2013 affected members of the Army and Air Force, which did not implement accession screening.
Editorial Comment
Using a laboratory-based case finding algorithm, this study initially identified 30 cases of prevalent HCV diagnoses in the 2020 active component military population occurring at fixed MTFs during the course of their military service. After correcting for cases which were referred to purchased care, the estimate of cases was revised to 68, which was only 12% of the expected number of infections (570) based on a previously established prevalence estimate of 43 per 100,000.11 This resulted in a corrected 2020 period prevalence of 5.2 diagnoses per 100,000. Of the 30 cases diagnosed at MTFs, 28 (93%) received medical therapy. Of the 28 who received medical therapy, 27 (96%) had attained SVR. The high estimates of HCV treatment and cure were apparently not affected by referral to purchased care. Identified cases were predominantly male, and the Army had the highest prevalence among the services. The disease showed a bimodal distribution in age with the highest prevalence in those over 41 and those aged 26–35. Only 3% of cases were symptomatic, and most cases did not have an identified source of transmission.
The estimated prevalence of chronic HCV infection is about 1% in the U.S. population,1 but the prevalence in the military has been previously estimated to be much lower, at 0.04%.11 This study reports an even lower HCV prevalence of 5.2 diagnoses per 100,000 service members, or 0.005%, identified at any time during their military service. The bimodal age distribution of HCV cases was similar to patterns seen in the general U.S. population.15 Roughly 50% of those with chronic HCV infection in the general population are aware of their diagnosis,16 compared to the much lower estimate of 12% in the active military in this study. In contrast, whereas only 49% of those with commercial insurance who are aware of their diagnosis received treatment,3 93% of service members diagnosed in MTFs received treatment. Additionally, attributed sources of infection appear to be vastly different in the military compared to the civilian population. However, this difference is likely due to a substantial reporting bias within the military population; illicit drug use is a punishable offense under military law, as is failure to disclose such use at time of accession into military service.
Strengths of this study include a large enumerated population and a detailed chart review of all subjects of interest in order to verify case results. However, several limitations should be considered when interpreting these findings. Most importantly, these estimates likely underestimate the true prevalence of HCV infection in the military due to the lack of asymptomatic screening in the Army and Air Force, as well as those Navy and Marine Corps service members who entered service prior to the implementation of accession screening for HCV infection in 2012.
Restricting laboratory surveillance of HCV to only those specimens which were ordered through ID and GI clinics since 2011 also resulted in underestimates. While the evaluation and treatment of HCV infection is generally restricted to these clinics, it is possible that some patients were diagnosed and treated in primary care clinics at MTFs or that some were diagnosed prior to 2011. The initial algorithm used to obtain these data was generated empirically by NMCPHC because 1) it was believed that most active component cases were treated in MTFs, and 2) the data available for assessing direct care was thought to be much more complete and of higher quality than that available for purchased care. However, due to concerns regarding the completeness of case capture from the original case-finding algorithm, an additional analysis of cases referred to purchased care was performed. This analysis suggested that only 44% of HCV cases were, in fact, diagnosed and treated in the direct care system. Although the data in the electronic medical records from purchased care were less complete and more difficult to access, sufficient documentation was available to assess the outcomes in this study. Nevertheless, the potential for misclassification of outcomes is greater in cases treated by purchased care compared to those treated by direct care due to the more limited data available.
Additionally, the NMCPHC algorithm does not include laboratory data from facilities which implemented the new electronic health record for the Military Health System, MHS GENESIS. The effect of this error is expected to be small because GENESIS was not implemented until late 2017 and had been less than 10% implemented by the end of 2020.17 In contrast, this study's use of period prevalence during the service members' military service may not be comparable to the point prevalence estimate from Brett-Major et al.
Although most laboratories currently perform reflex PCR testing for all positive HCV antibody tests, it is possible some service members had a positive HCV antibody test at a time when no PCR testing capability existed and thus never had a confirmatory PCR test. It should also be considered that the estimate of the total number of military HCV infections is based on a serosurvey among those with combat deployments from 2007 through 2010; therefore, this estimate may not be fully comparable to that obtained from the active component military population in 2020.
Another limitation was the potential for misclassification bias regarding potential exposures that result in HCV infection. High risk behaviors that can lead to HCV infection, such as intravenous drug use, are not permitted the Armed Forces and those who may have a history of such use may be reluctant to disclose this or other high-risk behaviors for fear of reprisal. While these concerns are present in the civilian population, they are likely heightened among military members because of additional legal consequences of reporting high risk behaviors. Finally, these results are not generalizable to populations outside of the U.S. military.
Given the recent changes in CDC and USPSTF recommendations for screening of nearly all adults aged 18–79 and the increasing incidence of HCV infection in younger individuals in the U.S. population, it is reasonable for the Army and Air Force to revisit their policies regarding accession screening. Comparison of identified HCV diagnoses before and after the Navy and Marine Corps implemented universal accession screening revealed that no cases were identified in those branches since this policy was initiated. This finding supports the argument that most HCV infections actually exist at time of entry into military service and that very few cases are identified after accession or occur during military service.
Another case for HCV screening is that it would mitigate the potential risk that HCV transmission poses to military operational requirements like the walking blood bank.18 Results of this study suggest that 88% of HCV infections are unrecognized and such persons pose the risk of HCV transmission if their blood is used for transfusions. HCV also poses risks to soldiers and health care workers rendering care in a combat environment where universal precautions cannot always be ensured. There is one case report of HCV transmission resulting from emergency transfusion during deployment, a rate of 2.1 per 1,000 persons.18
The counterargument against HCV screening within the U.S. military is that this population is at low risk for infection and for progression to symptomatic disease, both of which were demonstrated in this study. It is also noteworthy that the CDC recommendation for universal screening for HCV infection includes a caveat that it is not explicitly recommended in settings where the prevalence is less than 0.1%, although screening "may occur at the provider's discretion."9 The low expected prevalence of HCV infection at time of entry into service suggests that the initiation of screening would not result in a large impact on recruiting and, in fact, any recruits found to have HCV infection could be cured in a few months resulting in eligibility to enter the military service.
Future studies should review all HCV testing conducted within the Armed Forces (not just those ordered from ID or GI clinics) which would help to validate these results. Additionally, incidence studies would be helpful in determining rates of new infections, instead of prevalent diagnoses, as well as provide a more complete view of risk factors associated with infection.
Author affiliations: Department of Preventive Medicine & Biostatistics, Uniformed Services University, Bethesda, MD (Dr. Mancuso, Dr. Legg); Naval Medical Leader and Professional Development Command (NML&PDC), Bethesda, MD (Dr. Gleeson); Navy and Marine Corps Public Health Center, Portsmouth, VA (Mr. Seliga, Dr. Mahaney); General Dynamics Information Technology, Inc. (Dr. Mahaney).
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of Defense or the U.S. Government.
References
- Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69(3):1020–1031.
- Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: A meta-analysis. World J Hepatol. 18 2015;7(5):806–813.
- Isenhour C, Hariri S, Vellozzi C. Monitoring the hepatitis C care cascade using administrative claims data. Am J Manag Care. 2018;24(5):232–238.
- Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266.
- Safreed-Harmon K, Blach S, Aleman S, et al. The Consensus Hepatitis C Cascade of Care: Standardized reporting to monitor progress toward elimination. Clin Infect Dis. 2019;69(12):2218–2227.
- Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419.
- Ryerson AB, Schillie S, Barker LK, Kupronis BA, Wester C. Vital signs: Newly reported acute and chronic hepatitis C cases - United States, 2009-2018. MMWR Morb Mortal Wkly Rep. 2020;69(14):399–404.
- Chou R, Dana T, Fu R, et al. Screening for hepatitis C virus infection in adolescents and adults: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(13):1318.
- Hepatitis C Questions and Answers for Health Professionals. Centers for Disease Control and Prevention; 2020. Accessed 1 June 2021. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm
- Ballard T, Rohrbeck P, Kania M, Johnson LA. Transfusion-transmissible infections among U.S. military recipients of emergently transfused blood products, June 2006-December 2012. MSMR. 2014;21(11):2–6.
- Brett-Major DM, Frick KD, Malia JA, et al. Costs and consequences: Hepatitis C seroprevalence in the military and its impact on potential screening strategies. Hepatology. 2016;63(2):398–407.
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction 6130.03. Medical Standards for Appointment, Enlistment, or Induction in the Military Services. 6 May 2018.
- Office of the Secretary of the Navy. SECNAV INSTRUCTION 5300.30E Management of Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Infection in the Navy and Marine Corps. 13 August 2012.
- Department of Defense, Office of the Deputy Assistant Secretary of Defense for Military Community and Family Policy (ODASD (MC&FP)). 2019 Demographics Profile of The Military Community. Accessed 20 May 2021. https://download.militaryonesource.mil/12038/MOS/Reports/2019-demographics-report.pdf
- Centers for Disease Control and Prevention. Viral Hepatitis. Surveillance for Viral Hepatitis – United States, 2017 Updated. 2017. Updated 14 November 2019. Accessed June 1, 2021. https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm
- Yehia BR, Schranz AJ, Umscheid CA, Lo Re V, 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9(7):e101554.
- U.S. General Accountability Office. Electronic Health Records: DOD Has Made Progress in Implementing a New System, but Challenges Persist. Updated September 2021. Accessed Nov. 21, 2021.https://www.gao.gov/assets/720/716640.pdf
- Hakre S, Peel SA, O'Connell RJ, et al. Transfusion-transmissible viral infections among US military recipients of whole blood and platelets during Operation Enduring Freedom and Operation Iraqi Freedom. Transfusion. 2011;51(3):473–485.