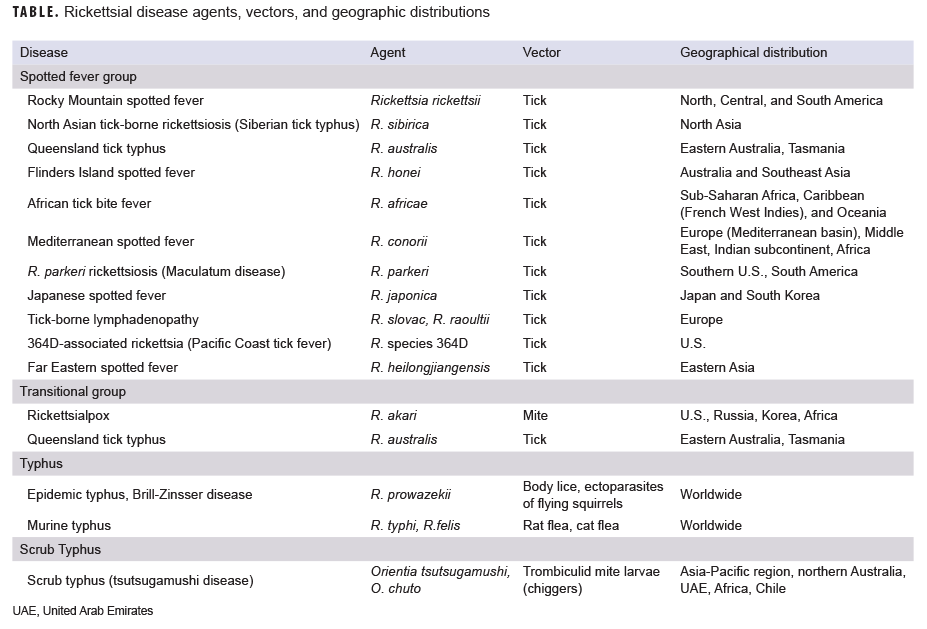
Rickettsial diseases are vector-borne bacterial infections that cause acute febrile illness throughout the world. They are spread via arthropod vectors including ticks, fleas, mites, and lice. They are caused by bacterial species of the genus Rickettsia and the closely related, but genetically distinct, genus Orientia. The Rickettsia and Orientia genera both encompass a large group of obligate intracellular, gram-negative bacteria. Species classified within the genus Rickettsia are generally divided into 4 groups (i.e., clades). The ancestral group includes the tick-borne agents Rickettsia bellii and R. canadensis but does not contain pathogens that cause human disease. The spotted fever group (SFG) comprises more than 30 species and includes the etiologic agents for Rocky Mountain spotted fever (RMSF), African tick-bite fever (ATBF), and Mediterranean spotted fever (MSF). The typhus group includes the pathogens that cause epidemic and murine typhus, while the transitional group includes agents that cause rickettsialpox and Queensland tick typhus.1,2 Scrub typhus is caused by 2 known Orientia species Orientia tsutsugamushi (formerly R. tsutsugamushi) and the relatively newly discovered O. chuto (Table).3 Rickettsial-related diseases are caused by the bacterial species of the genera Ehrlichia, Anaplasma, Neoehrlichia, and Neorickettsia; however, for the purposes of this review, infections caused by these species are not discussed further in this article (Table).
Despite the widespread distribution of rickettsial diseases worldwide, they are frequently overlooked as a cause of illness and/or misdiagnosed. This is partly due to the non-specific nature of the early symptoms of rickettsial diseases, which frequently present as undifferentiated febrile illness that is often indistinguishable from other infectious diseases, especially those common in tropical and subtropical regions (e.g., malaria, dengue fever, leptospirosis).4 This has contributed to the underdiagnosis of these diseases and the likely significant underestimation of their incidence.1,2
Rickettsial diseases have had a significant impact on public health and have been a significant cause of morbidity and mortality in both civilian and military populations.5 In addition, rickettsial pathogens continue to emerge and reemerge as causes of illness throughout the world.6 Reported incidences of several rickettsial diseases, notably scrub typhus in the Asia/Pacific region and SFG rickettsioses in the U.S., have increased substantially.7 Understanding of the epidemiology of rickettsial diseases continues to evolve as new information accumulates about the expanding geographic distribution of the causative pathogens,8 the emergence of antibiotic-resistant strains,9 and the discovery of new species in the genera Rickettsia and Orientia. To provide a summary of this and other practical information on rickettsial diseases, a brief review of epidemiologic and clinical characteristics of specific rickettsial and related diseases is provided, with an emphasis on their historical and potential future impact on U.S. military forces.
Scrub typhus
Scrub typhus is a potentially fatal acute febrile disease transmitted by larval mites (i.e., "chiggers"), primarily of species of the genus Leptotrombidium that are infected by the obligate intracellular bacteria O. tsutsugamushi. The mite serves as both the vector and the reservoir for the disease.1,10
Once considered endemic only to central, eastern, and Southeast Asia as well as northern Australia and islands in the Pacific and Indian oceans (i.e., the tsutsugamushi triangle), case reports of scrub typhus from South America,11,12 Africa, the Middle East, and Europe8 have provided substantial evidence that the geographic range of scrub typhus is more extensive than previously thought. In 2010, a new species (O. chuto) was described in an Australian tourist who contracted scrub typhus in Dubai.3 Scrub typhus does not occur in the U.S. except when diagnosed in travelers who have returned from endemic areas.1
Globally, scrub typhus is a leading cause of febrile disease.6 It has been estimated that over a million scrub typhus cases occur each year and that a billion people are at risk of infection.10 Several indicators point to an overall global increase in the incidence of scrub typhus. The 5 countries with established scrub typhus surveillance systems (China, Japan, South Korea, Taiwan, and Thailand) all have reported increasing incidence of this disease over the past 10–15 years.7 Additionally, between 2007–2017, at least 22 scrub typhus outbreaks have been documented in endemic areas, with India accounting for almost two-thirds (14/22) of reported outbreaks.13 However, it is unclear whether the increases in the incidence of diagnosed cases or in documented outbreaks reflect an actual increase in disease incidence or whether they are the result of enhanced awareness of the disease, increased surveillance, and/or improved case ascertainment related to improved diagnostic capabilities.7,13
Symptoms of scrub typhus begin 7–10 days after the bite of an infected mite. Classic symptoms include headache, fever, and a generalized maculopapular rash. A necrotic lesion known as an eschar may also develop around the site of the bite. Typically, the eschar begins as a vesicle and progresses to a central brown/black crust after several days. Less common symptoms include myalgia, altered mental status, and lymphadenopathy.1,13
A current or past scrub typhus infection can be identified by the presence of specific antibodies (immunoglobulin M [IgM] and G [IgG]) against scrub typhus group orientiae. A single sample with a positive IgM is associated with acute infection, while detection of IgG antibodies does not adequately differentiate between current or past infection. Seroconversion or a 4-fold rise in IgG titer using paired serum samples (acute and convalescent) are the preferred method for diagnosing scrub typhus. Historically, laboratory diagnosis of scrub typhus has mainly relied on serologic tests, particularly the indirect immunofluorescence assay (IFA). However, the IFA is an imperfect gold standard because of its high cost, the need for paired sera, the need for substantial training to perform the test, and interoperator variability in result interpretation. Increasingly, anti-Orientia IgM- and IgG-based rapid diagnostic tests and enzyme-linked immunosorbent assays (ELISAs) are replacing subjective IFAs. Molecular techniques such as real-time polymerase chain reaction (RT-PCR) can also be useful in scrub typhus diagnosis and the confirmation of serological results.14
Scrub typhus is generally easily treatable with doxycycline if diagnosed early. A recent Cochrane review demonstrated that tetracycline, azithromycin, and rifampicin are also effective antibiotics for scrub typhus treatment.15 In untreated patients, the median mortality rate for scrub typhus is 6% (range: 0–70%), while a recent review of treated scrub typhus reported a median mortality of 1.4% (range: 0–33.3%).7 Doxycycline has also been used as prophylaxis for scrub typhus.16
The possibility of antibiotic-resistant scrub typhus has been a significant concern since the 1990s when multiple reports of antibiotic resistance emerged from Thailand.17 Subsequently, in vivo, in vitro, and clinical data have supported the existence of strains of O. tsutsugamushi resistant to conventional antibiotic therapy. Further research, including clinical trials and laboratory-based studies, are warranted to definitively determine the existence, distribution, and extent of antibiotic-resistant typhus.9,13
No vaccine for scrub typhus exists. The development of a prophylactic vaccine for scrub typhus has been a public health priority for decades. Significant obstacles, including extensive antigenic diversity and the short duration of immune protection following naturally acquired scrub typhus infection, have stymied successful vaccine development.18,19 Current scrub typhus prevention methods are primarily focused on vector control and reducing exposure to chiggers. The latter method includes wearing long pants tucked into boots or socks, long sleeved shirts, and boots or other closed-toed shoes. The use of both skin repellent and repellent-treated clothing is recommended. Effective skin repellent should contain 20–50% DEET (N,N-diethyl-meta-toluamide), while permethrin is an effective clothing impregnant.
Military impact
Scrub typhus was a significant cause of acute febrile illness among Allied troops in the Pacific during World War II, causing approximately 18,000 cases; over 6,000 cases and 243 deaths were reported by U.S. Armed Forces.13 During the Vietnam War, scrub typhus was estimated to cause 20–30% of cases of fevers of unknown origin in U.S. troops. More recently, Camp Fuji in Japan has been the site of multiple outbreaks among U.S. military members, with the most recent outbreaks reported in U.S. Marines in 2000 and 2001.5 Sporadic cases of scrub typhus have also occurred in Australian military members during training in Northern Queensland, Australia, especially at a training site called Cowley Beach.13 In 1996, the number of scrub typhus cases at Cowley Beach prompted the Australian military to recommend doxycycline prophylaxis for military members training at that location.13 A large 2011 outbreak (45 cases among 124 exposed; attack rate of 36%) in Australian infantry and support staff training at Cowley Beach raised concerns that a doxycycline resistant strain of O. tsutsugamushi was responsible for the outbreak. However, further laboratory analysis demonstrated that the outbreak strain was susceptible to doxycycline, indicating that failure to adhere to the doxycycline prophylaxis protocol was a more likely explanation for the outbreak.16 This episode clearly demonstrates that adherence to protective measures, including prophylaxis protocols, must be a priority.
Given the endemicity of scrub typhus in countries where significant numbers of U.S. military personnel are deployed or train (e.g., South Korea, Japan, Thailand), the emergence of antibiotic resistance in these areas, and the historical impact of scrub typhus on military operations, continued focus on allocating resources to maintain robust research efforts towards vaccine development, improved laboratory diagnostics, and enhanced surveillance are warranted.20
Murine (endemic) typhus
Murine typhus, also known as fleaborne typhus, is a rickettsial zoonosis caused by R. typhi. It is transmitted mainly by the rat flea (Xenopsylla cheopis), and human infection can occur through flea bites, infected flea feces scratched into broken skin (i.e., a flea bite wound), or via other mucous membranes or inhalation. The primary reservoirs of R. typhi are the roof rat (Rattus rattus) and Norway rat (Rattus norvegicus).1 However, in the U.S., opossums and cats are important reservoirs of infection, and the cat flea has been identified as the principal vector.21
Murine typhus occurs at endemic levels throughout the world, especially in tropical and subtropical seaboard regions. Although murine typhus is no longer a nationally notifiable disease in the U.S., it is reportable in 14 states. It is most frequently reported in California, Hawaii, and Texas, with the majority of reported cases occurring in Texas.22
R. typhi infection usually produces a mild or self-limiting illness. Symptoms are generally non-specific and include fever, headache, and myalgia. Rash occurs with varying frequency. Murine typhus is commonly misdiagnosed when rash is absent or if atypical symptoms, such as gastrointestinal manifestations, are prominent.23 Severe complications are rare, but, where present, can cause meningoencephalitis, pneumonia, shock, renal failure, myocarditis, endocarditis, and splenic rupture. The primary treatment for murine typhus is doxycycline. Murine typhus has an overall case fatality rate of between 1–4%.24
Military impact
During World War II, 787 cases of murine typhus were reported in U.S. military members; of these, 497 cases occurred within the continental U.S., mostly in the southeast.5 The reported mortality rate was 1.9%.20 Although relatively few cases were reported during the Vietnam War, serologic studies indicated that approximately 10–15% of fevers of unknown origin could be attributed to murine typhus, making it second only to malaria as a cause of febrile disease during this conflict.20 Over the past 2 decades, murine typhus has been infrequently diagnosed in U.S. service members; on average, less than 2 confirmed cases a year are reported.25
Deployment to endemic regions on peacekeeping or humanitarian missions could pose a substantial risk of exposure to military personnel since overcrowding and poor public health and sanitation measures (such as those that occur during natural disasters and in refugee centers) provide ideal conditions for transmission of murine typhus.
Epidemic typhus
Epidemic typhus (also known as louseborne typhus or camp fever) is an acute febrile illness caused by R. prowazekii. R. prowazekii is transmitted by the human body louse (Pediculus humanus). Transmission dynamics are similar to murine typhus in that human infection occurs when infected louse feces are inhaled or enter the body through broken skin (typically through scratching the louse bite).1,2
A second strain of R. prowazekii has been identified in southern flying squirrels (Glaucomys volans), which has caused sporadic human cases in rural and suburban areas of the eastern U.S.26,27 Disease resulting from this method of transmission is called sylvatic epidemic typhus or sylvatic typhus.28 The cycle of infection involves secondary transmission to humans from flying squirrels and their ectoparasites, but the mechanism by which R. prowazekii is transmitted to humans remains unclear. Although infection is generally sporadic, clusters have been reported in cases of repeated and prolonged close exposure to flying squirrels and their nests.28
The incubation period of epidemic typhus is typically between 7 and 14 days. Onset of symptoms is sudden and includes high fever, headache, tachypnea (abnormally rapid breathing), and myalgia. Rash is also a frequent symptom and generally starts as small pink macules that spread over the trunk and become dark and maculopapular. The case-fatality ratio can reach 60% among untreated patients, decreasing to below 5% with appropriate antibiotic treatment and supportive care.29 R. prowazekii infection can be reactivated in humans years or decades after primary infection because of a waning immune system. This mild recrudescence of epidemic typhus is called Brill-Zinsser disease.29 Cases of Brill-Zinsser disease have been reported in Europe, the U.S., and Canada. Doxycycline is the recommended treatment for both primary cases of epidemic typhus and Brill-Zinsser disease.1,29
Military impact
R. prowazekii caused major outbreaks of disease in many conflicts up to and including World War I. As an example of the magnitude of morbidity and mortality caused by this agent, in the period between 1917–1925 in eastern Europe and Russia, up to 25 million cases and 3 million deaths were suspected to be due to epidemic typhus.30
During and immediately after World War II, hundreds of thousands of cases occurred in civilian populations in Korea, Japan, Germany, Egypt, and French North Africa.5 However, because the U.S. military implemented the Joint U.S. Typhus Commission recommendations, which included the use of dichloro-diphenyl-trichloroethane (DDT) for louse control, prophylactic immunization by the Cox-type vaccine, and other preventive measures, it experienced only 104 cases and no deaths.5 These measures, along with newer insecticides, also proved effective during the Korean conflict, virtually eliminating cases of epidemic typhus in U.S. troops (1 case was reported).5 However, during the Korean conflict, epidemic typhus caused significant morbidity and mortality among South Korean soldiers and civilians, with approximately 32,000 cases and 6,000 deaths.5 No cases of epidemic typhus were reported in U.S. military members during the Vietnam conflict.5
Since the 1990s, epidemic typhus has reemerged. Most epidemic typhus cases are reported from Africa and Central and South America, particularly during the winter and spring, when hygiene may be compromised. In 1997, a significant outbreak occurred in Burundi during the civil war. The cases were associated with refugee camps.31 As with murine typhus, this illustrates that U.S. military members supporting peacekeeping and humanitarian missions have the potential for exposure to R. prowazekii.5,29
SFG rickettsioses
SFG rickettsiae are all transmitted by ticks (Table). These organisms infect tick species throughout the world. The SFG rickettsiae vary in pathogenicity and cause disease with a spectrum of severity ranging from those with significant morbidity and mortality (e.g., R. rickettsii) to those with more benign manifestations (e.g., R. parkeri, R. species 364D).1,2,32 The more common and pathogenic SFG rickettsioses are briefly discussed below.
RMSF is caused by R. rickettsii. Despite its name, RMSF is endemic in parts of North, Central, and South America. In the U.S., RMSF is transmitted by the American dog tick (Dermacentor variabilis)in the southeast and south central states and the Rocky Mountain wood tick (Dermacentor andersoni) in the western mountainous states. In Central and South America, transmission occurs via multiple species within the genus Amblyomma, including the cayenne tick (Amblyomma cajennense).1,32
The incubation period for RMSF averages 7 days but ranges from 3 to 12 days.32 A shorter incubation period presages a more severe infection. Onset is abrupt, with severe headache, fever, chills, malaise, and myalgia. Between the second and fourth day of fever, most patients develop a rash on the wrists, ankles, palms, soles, and forearms that rapidly extends to the neck, face, buttocks, and trunk. Initially macular and pink, the rash becomes maculopapular and darker, and the lesions subsequently become petechial and can coalesce to form large hemorrhagic areas that later ulcerate.32
RMSF is the most severe and most frequently fatal rickettsial disease in the U.S. Fatality rates range from 5–10%, depending upon the timing of initiation of treatment; fatality rates increase to 40–50% if treatment is delayed until after day 8. As with all tick-borne rickettsial disease, the Centers for Disease Control and Prevention (CDC) recommends doxycycline as the drug of choice for treatment, which should be initiated immediately in persons with signs or symptoms suggestive of RMSF.32
The rickettsial pathogens most likely to be encountered during travel outside the U.S. include R. africae (ATBF) and R. conorii (MSF).1,32 ATBF is a zoonotic disease transmitted by ticks of the genus Amblyomma in sub-Saharan Africa. Usual symptoms include fever, 1 or more inoculation eschars, and regional lymphadenopathy. Rash is frequently absent and complications are uncommon. MSF, also called boutonneuse fever, is transmitted by the infected brown dog tick (Rhipicephalus sanguineus). MSF is endemic in the Mediterranean, the Indian subcontinent, regions around the Black Sea, and the sub-Saharan African countries. Symptoms include headache, fever, and a maculopapular rash. An eschar is also commonly seen at the site of the tick bite. Doxycycline is the first-line treatment of choice.
In the U.S., the annual incidence of SFG rickettsioses has increased substantially. Between 2000 and 2016, the annual incidence of SFG increased more than 7-fold from 1.7 cases per 1 million persons to 13.2 cases per 1 million persons.32 These data are subject to two main limitations. Before 2010, only RMSF was a notifiable disease. However, because serologic assays developed for the diagnosis of RMSF may react non-specifically with antigens of less pathogenic species, the category was changed to the more general SFG rickettsioses. This change in classification may have contributed to the increase in SFG incidence.32
In addition, concerns regarding the magnitude of this increase have prompted an examination of the underlying data reported to CDC, which highlighted some potential issues that could affect the accuracy of SFG incidence estimates in the U.S. In brief, in the U.S., all SFG rickettsioses, including RMSF are nationally notifiable diseases. The CDC is notified of SFG cases through 2 passive surveillance systems: the National Notifiable Diseases Surveillance System (NNDSS) and Tick-borne Rickettsial Disease case report forms. SFG rickettsioses are identified using the Council of State and Territorial Epidemiologist case criteria, which include serologic methods (some of which are of limited interpretability [e.g., IFA]) as supportive evidence and non-specific laboratory criteria to support diagnosis.33 To illustrate the implications of this practice, CDC performed a review of cases with illness onset reported during 2010–2015. CDC determined that of 16,807 reported cases, only 167 (1.0%) met the confirmed case definition and the remaining 16,640 (99.0%) met the probable case definition.33 The most common laboratory criteria used to support probable cases was elevated IgG antibody titer by IFA. The use of IFA is problematic because antibodies to SFG Rickettsia persist for months following infection; a single antibody titer may represent prevalent (previous) infection rather than incident (acute) infection.33 It would be preferable for a greater percentage of cases to meet the more stringent criteria for a confirmed SFG case (e.g., a 4-fold change in anti-SFG IgG antibody titers in paired specimens, PCR, immunohistochemistry, or culture). However, the majority of probable cases were not confirmed because of incomplete serologic testing.33 This investigation demonstrated that the quality of passive surveillance data depends on provider awareness and use of appropriate diagnostic tests coupled with timely reporting and documentation of epidemiologic factors associated with the reported case.33 Moreover, this investigation highlighted the need for a complete and thorough understanding of the case definitions and knowledge of the relative proportions of confirmed and probable cases in order to appropriately interpret estimated incidence rates of SFG rickettsioses.33
Military impact
Multiple studies have demonstrated that U.S. military personnel are at significant risk of exposure to SFG rickettsioses. This risk can be due to residence in or deployment to endemic regions or from field training in areas where infected ticks live. As the most severe SFG rickettsiosis, RMSF may be the rickettsial disease with the most significant consequences for the U.S. military, given its prevalence in areas where military training takes place. Epidemiologic studies have demonstrated SFG rickettial infections (including R. rickettsia) in several military units conducting training exercises in Arkansas and Virginia34 and among male personnel in combat occupations stationed in South Korea.35 One of the most significant outbreaks of SFG rickettsiosis occurred in 1992 among members of U.S. Army 82nd Airborne Division conducting a training mission in Botswana. Approximately 50% of the unit were diagnosed with ATBF.5
Transitional group rickettsioses
Briefly, pathogenic rickettsial species in the transitional group include R. akari, the causative agent of rickettsialpox, and R. australis, which causes Queensland tick typhus (Table). Rickettsialpox is transmitted by mites, while Queensland tick typhus is transmitted by Ixodes holocyclus ticks in Australia. Rickettsialpox occurs in many areas of the U.S., Russia, Korea, and Africa and is generally seen in urban areas.1 Common symptoms include fever, vesicular rash, and eschar. Rickettsialpox is a mild, self-limiting condition, and no deaths from this disease have been reported.1
Queensland tick typhus also presents with fever and maculopapular rash, and, less commonly, an eschar and associated regional lymphadenopathy can occur.1 While Queensland tick typhus is also generally a mild disease, both severe and fatal cases have occurred.1 Because of the mild presentation of both diseases, the impact on military forces would likely be relatively limited compared to more pathogenic rickettsial diseases.
Editorial Comment
Deployment of troops to endemic areas and exposure during humanitarian and peacekeeping missions will ensure that rickettsial diseases will remain a threat to military personnel. Unfortunately, rickettsial infections are not routinely diagnosed by most military medical providers, which is why they continue to pose a threat. Providers need to remain vigilant in considering rickettsial diseases during their diagnostic workup of military members who live, work, or train in rickettsial-endemic areas. While prophylaxis and personal protective measures can be effective, the necessary command support is required to ensure that these measures are adhered to, or they will not be effective.
References
- Blanton LS. The rickettsioses: a practical update. Infect Dis Clin North Am. 2019;33(1):213–229.
- Abdad MY, Abou Abdallah R, Fournier PE, Stenos J, Vasoo S. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol. 2018;56(8):1–10.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48(12):4404–4409.
- Van Eekeren LE, de Vries SG, Wagenaar JFP, Spijker R, Grobusch MP, Goorhuis A. Under-diagnosis of rickettsial disease in clinical practice: a systematic review. Travel Med Infect Dis. 2018;26:7–15.
- Bavaro MF, Kelly DJ, Dasch GA, Hale BR, Olson P. History of U.S. military contributions to the study of rickettsial diseases. Mil Med. 2005;170(4 suppl):49–60.
- Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11(11):e0006062.
- Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11(9):e0005838.
- Jiang J, Richards AL. Scrub typhus: no longer restricted to the tsutsugamushi triangle. Trop Med Infect Dis. 2018;3(1):E11.
- Kelly DJ, Fuerst PA, Richards AL. The historical case for and the future study of antibiotic-resistant scrub typhus. Trop Med Infect Dis. 2017;2(4):E63.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16(5):429–436.
- Weitzel T, Aylwin M, Martinez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Weitzel T, Dittrich S, Lopez J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375(10):954–961.
- Luce-Fedrow A, Lehman ML, Kelly DJ, et al. A review of scrub typhus (Orientia tsutsugamushi and related organisms): then, now, and tomorrow. Trop Med Infect Dis. 2018;3(1):E8.
- Paris DH, Dumler JS. State of the art of diagnosis of rickettsial diseases: the use of blood specimens for diagnosis of scrub typhus, spotted fever group rickettsiosis, and murine typhus. Curr Opin Infect Dis. 2016;29(5):433–439.
- El Sayed I, Liu Q, Wee I, Hine P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2018;9:CD002150.
- Harris PNA, Oltvolgyi C, Islam A, et al. An outbreak of scrub typhus in military personnel despite protocols for antibiotic prophylaxis: doxycycline resistance excluded by a quantitative PCR-based susceptibility assay. Microbes Infect. 2016;18(6):406–411.
- Watt G, Chouriyagune C, Ruangweerayud R, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348(9020):86–89.
- Valbuena G, Walker DH. Approaches to vaccines against Orientia tsutsugamushi. Front Cell Infect Microbiol. 2012;2:170.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375(10):913–915.
- Kelly DJ, Richards AL, Temenak J, Strickman D, Dasch GA. The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 2002;34(suppl 4):s145–s169.
- Blanton LS, Idowu BM, Tatsch TN, Henderson JM, Bouyer DH, Walker DH. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95(2):457–461.
- Basra G, Berman MA, Blanton LS. Murine typhus: an important consideration for the nonspecific febrile illness. Case Rep Med. 2012;2012:134601.
- Blanton LS, Vohra RF, Bouyer DH, Walker DH. Reemergence of murine typhus in Galveston, Texas, USA, 2013. Emerg Infect Dis. 2015;21(3):484–486.
- Dumler JS, Taylor JP, Walker DH. Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA. 1991;266(10):1365–1370.
- Stidham RA, von Tersch RL, Batey KL, Roach C. Case report: probable murine typhus at Joint Base San Antonio, TX. MSMR. 2015;22(8):13–16.
- Duma RJ, Sonenshine DE, Bozeman FM, et al. Epidemic typhus in the United States associated with flying squirrels. JAMA. 1981;245(22):2318–2323.
- Reynolds MG, Krebs JS, Comer JA, et al. Flying squirrel-associated typhus, United States. Emerg Infect Dis. 2003;9(10):1341–1343.
- Chapman AS, Swerdlow DL, Dato VM, et al. Cluster of sylvatic epidemic typhus cases associated with flying squirrels, 2004–2006. Emerg Infect Dis. 2009;15(7):1005–1011.
- Bechah Y, Capo C, Mege JL, Raoult D. Epidemic typhus. Lancet Infect Dis. 2008;8(7):417–426.
- Angelakis E, Bechah Y, Raoult D. The History of Epidemic Typhus. Microbiol Spectr. 2016;4(4).
- Raoult D, Ndihokubwayo JB, Tissot-Dupont H, et al. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet. 1998;352(9125):353–358.
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65(2):1–44.
- Binder AM, Nichols Heitman K, Drexler NA. Diagnostic methods used to classify confirmed and probable cases of spotted fever rickettsioses—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2019;68(10):243–246.
- Sanchez JL, Candler WH, Fishbein DB, et al. A cluster of tick-borne infections: association with military training and asymptomatic infections due to Rickettsia rickettsii. Trans R Soc Trop Med Hyg. 1992;86(3):321–325.
- Jiang J, Myers TE, Rozmajzl PJ, et al. Seroconversions to rickettsiae in US military personnel in South Korea. Emerg Infect Dis. 2015;21(6):1073–1074.