Abstract
Measles, mumps, rubella, and varicella (MMR/V) are highly communicable infectious diseases whose causative agents are spread through contact with contaminated surfaces or airborne droplets. Individuals at highest risk for MMR/V infections include infants; unvaccinated or inadequately vaccinated persons; individuals living in communities with low vaccination rates or in crowded, unsanitary conditions; and persons with compromised immune systems. Between 1 Jan. 2016 and 30 June 2019, there were 5 confirmed measles cases and 64 confirmed mumps cases among all Military Health System (MHS) beneficiaries. During this period, no cases of measles were reported among U.S. service members. There were 29 confirmed mumps cases among service members during the surveillance period; 2 cases occurred in 2016, 17 in 2017, 5 in 2018, and 5 in the first 6 months of 2019. There were 6 confirmed rubella cases among all MHS beneficiaries. Among service members, there were 39 confirmed cases of varicella during the surveillance period; 9 cases occurred in 2016, 11 in 2017, 11 in 2018, and 8 in the first 6 months of 2019. Recent trends in MMR/V in both military and civilian populations in the U.S. highlight the importance of primary and booster vaccinations.
What Are the Findings?
In the 3.5-year period, the confirmed cases of varicella, mumps, rubella, and measles among service members numbered 39, 29, 1, and 0, respectively. Among non-service member beneficiaries, the counts of cases were similar, numbering 69, 35, 5, and 5, respectively. These low case counts confirm the effectiveness of the respective vaccine components among the large MHS beneficiary population.
What Is the Impact on Readiness and Force Health Protection?
The methodical use of the MMRV vaccine among new service members eliminates the associated morbidity for the protected individuals and the potential for outbreaks of these diseases, which can impair the readiness of military units. A similar beneficial impact among non-service member beneficiaries reduces the health care burden on the medical infrastructure that supports force readiness.
Background
Measles, mumps, rubella, and varicella (MMR/V) were common in the U.S. before the introduction of licensed vaccines. Measles vaccine was introduced in 1963, mumps vaccine in 1967, rubella vaccine in 1969, and varicella vaccine in 1995.1 Since then, these vaccines have been important components of routine pediatric preventive care. Individuals at highest risk for MMR/V infections include infants (because they are too young to be vaccinated); unvaccinated or inadequately vaccinated persons; individuals living in communities with low vaccination rates or in crowded, unsanitary conditions; and persons with compromised immune systems.2 Although the numbers of cases of MMR/V declined dramatically in the U.S. after vaccine implementation, outbreaks of these diseases occur sporadically. Between 1 Jan. 2019 and 1 Oct. 2019, a total of 1,249 measles cases and 22 measles outbreaks were reported to the Centers for Disease Control and Prevention (CDC) from 31 states.3 The number of measles cases reported during this period represents the greatest number of cases reported in a calendar year in the U.S. since 1992; the majority of these cases occurred among individuals who were unvaccinated.3 Overall, approximately 10% of those who contracted measles during this period were hospitalized.3 Eighty-one of the measles cases reported so far in 2019 were imported from other countries.3 In 2016, 2017, and 2018, totals of 86, 120, and 372 cases of measles were reported in the U.S., respectively.4
Mumps outbreaks continue to occur in the U.S., even among vaccinated individuals and in areas with high vaccination rates.5 Two doses of the measles, mumps, and rubella (MMR) vaccine (which contains the live attenuated Jeryl Lynn mumps vaccine strain) are 88% effective at protecting against mumps.6 When mumps infection does occur among vaccinated individuals, the illness is usually less severe; moreover, mumps outbreaks tend to be of limited size and duration in communities with high vaccination rates.7,8 In the U.S., there has been an ongoing resurgence in mumps cases that began with a series of outbreaks on university campuses in 2006.9 More recently, between 1 Jan. 2016 and 30 June 2017, U.S. health departments reported 150 outbreaks (9,200 cases) associated with schools, universities, athletics teams and facilities, households, church groups, workplaces, and large parties and events.8 In 2018, a total of 2,251 mumps cases were reported to CDC.4 Between 1 Jan. and 13 September 2019, a total of 2,363 cases were reported from 47 states and the District of Columbia.8
Rubella is the leading vaccine-preventable cause of birth defects worldwide; infection in pregnant women may lead to fetal death or congenital defects.10 In the U.S., rubella and the associated congenital rubella syndrome were documented as eliminated in 2004.10 Elimination in this context means that the disease is no longer spread year-round in the U.S. or the Americas region.10 Although rubella has been eliminated in the U.S., it remains endemic in many other parts of the world. During 2016–2017, fewer than 10 people in the U.S. were reported as rubella cases.11 All people who were reported as cases of rubella infection since 2012 had evidence that they acquired the infection when they were living or traveling outside the U.S.12
Data on the number of chickenpox (varicella) outbreaks that occur each year in the U.S. are unavailable. Although chickenpox outbreaks are not notifiable at the national level, states are encouraged to report them to CDC annually.13,14 States are also encouraged to conduct ongoing varicella surveillance to monitor vaccine impact on morbidity. Forty states were carrying out case-based varicella surveillance as of 2017.14 Passive surveillance data collected between 1 Aug. 2015 and 7 Jan. 2017 indicate that 49 jurisdictions reported 89 outbreaks of varicella (1,030 cases), the majority of which occurred in schools and day care settings (57%).13 Available passive surveillance data suggest that varicella outbreaks during 2005–2012 decreased in size (number of varicella cases per outbreak) and duration15; however, no U.S. reports on varicella outbreak trends are available for more recent time periods.
In the U.S., school vaccination requirements have been shown to be a very effective strategy for achieving and maintaining high varicella vaccination coverage among school-aged children.16 The single-dose varicella vaccination program begun in 1996 was associated with significant decreases in disease burden from varicella.17 However, outbreaks of varicella remained a problem even among school populations with high single-dose coverage.18 In 2007, a universal 2-dose varicella childhood vaccine schedule with a catch-up vaccination for susceptible (i.e., only 1 dose of varicella vaccine) children, adolescents, and adults was recommended to improve protection and further decrease varicella cases and outbreaks.18 Since these more recent recommendations were implemented, additional declines in varicella-related outpatient visits and hospitalizations have been documented.19
Because of the public health and military operational consequences of MMR/V infections, evidence of immunity to these viruses is required for service members. Certain military environments such as barracks and ships are conducive to person-to-person spread of diseases such as MMR/V. Furthermore, many service members are sent to overseas locations where the likelihood of exposure to these viruses is elevated. For example, from late Dec. 2018 through early April 2019, 28 U.S. Navy and Marine Corps members aboard the USS Fort McHenry were diagnosed with viral parotitis, which the Navy later described as probable cases of mumps.20 More recently, in late July 2019, several Army paratroopers showed symptoms of mumps while in Italy. One of these soldiers later tested positive for mumps while on temporary duty in Germany.21 The infected soldier was up to date on all of his vaccinations, including MMR.22 In response to this occurrence, Army medical staff administered the MMR vaccine to about 200 soldiers based in Italy.21,22
In October 2017, the MSMR reported on MMR/V diagnoses among service members and other Military Health System (MHS) beneficiaries.23 The current analysis provides updated summaries of the numbers, trends, and demographics of diagnoses of these diseases among these MHS populations.
Methods
The surveillance period was 1 Jan. 2016 through 30 June 2019. The surveillance population included all individuals who were MHS beneficiaries (i.e., active and reserve/guard component service members, retired service members, family members and other dependents of service members and retirees, and other authorized government employees and family members) who accessed care through either a military medical facility/provider or a civilian facility/provider (if paid for by the MHS). It is Department of Defense (DOD) policy that cases of MMR/V (as well as many other diseases of public health importance) be reported electronically through military health channels for surveillance purposes.24 Conditions covered by this policy are referred to as reportable medical events (RMEs). All data used to ascertain cases for this analysis were derived from the electronic records of the Defense Medical Surveillance System (DMSS).
For this analysis, a "confirmed" case was defined as an individual identified through an RME of MMR/V that was described as confirmed by meeting specified laboratory or epidemiologic criteria.25–28 Because reporting policy for RMEs of varicella was limited to active duty service members before 2017, results pertaining to confirmed varicella cases in 2016 were limited to those reported among members of the active and reserve components.24
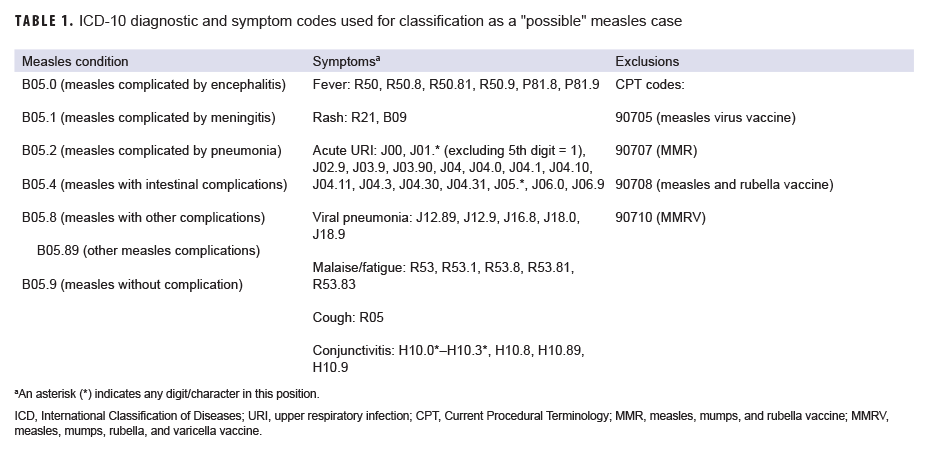
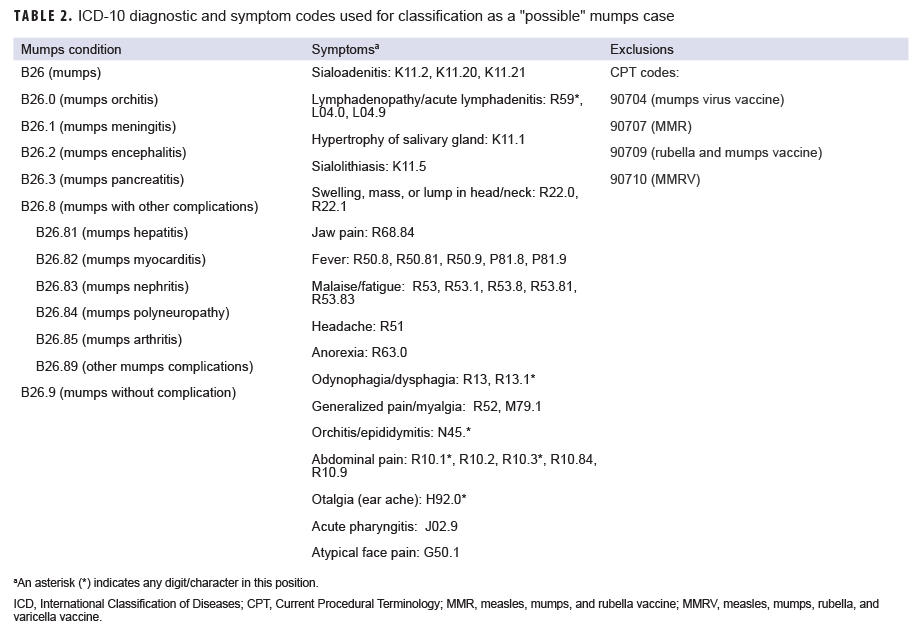
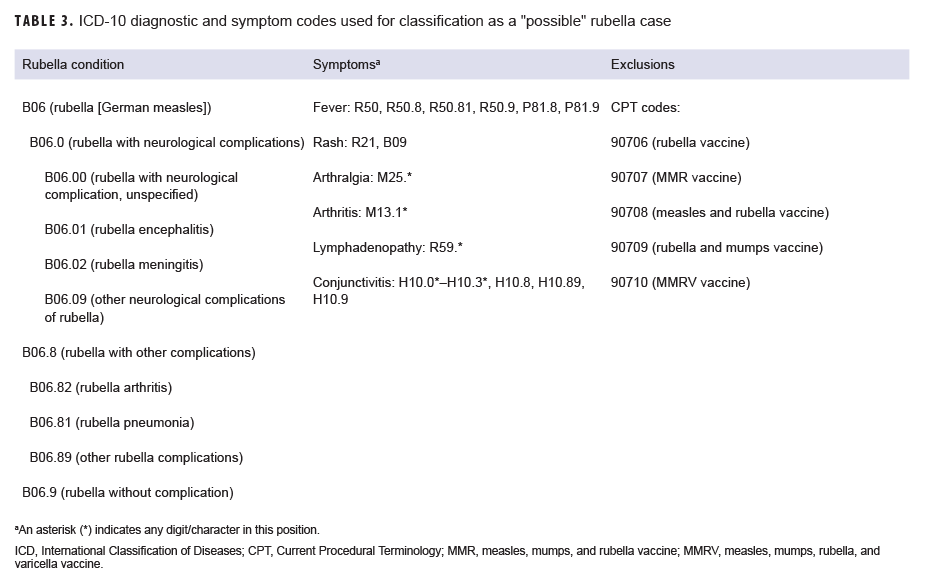
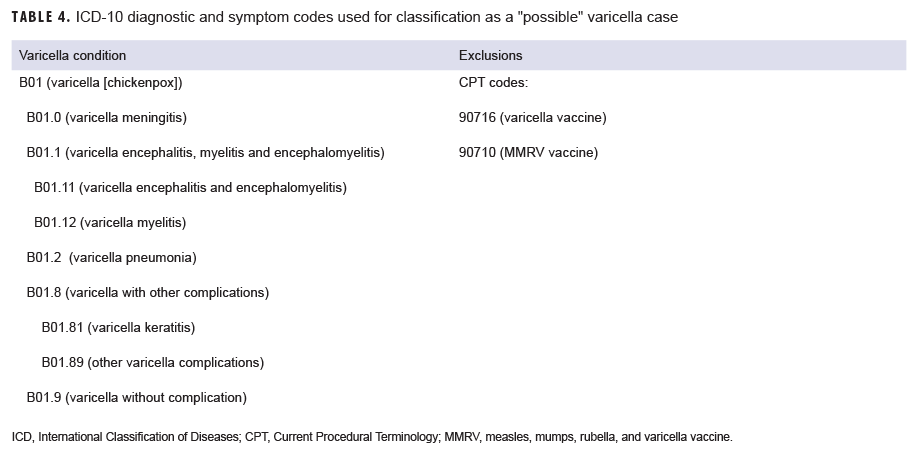
A "possible" case was defined as 1) an RME of MMR/V without laboratory or epidemiologic confirmation or 2) a record of an inpatient or outpatient medical encounter with a diagnosis of measles (International Classification of Diseases, 10th Revision [ICD-10]: B05.0, B05.1, B05.2, B05.4, B05.8, B05.89, B05.9), mumps (ICD-10: B26*), rubella (ICD-10: B06*), or varicella (ICD-10: B01.0, B01.11, B01.12, B01.2, B01.81, B01.89, B01.9) in the primary diagnostic position (Tables 1–4). "Possible" MMR cases were also required to have an associated symptom code listed in another diagnostic position (Tables 1–3). Encounters were excluded if there was either 1) a record of MMR/V vaccine administration or a positive test for serologic immunity to MMR/V within 7 days before or after the encounter date or 2) an ICD-10 diagnosis or a Current Procedural Terminology (CPT) code indicating MMR/V vaccination recorded for the same encounter as the diagnosis of MMR/V (Tables 1–4).
Results
Confirmed cases
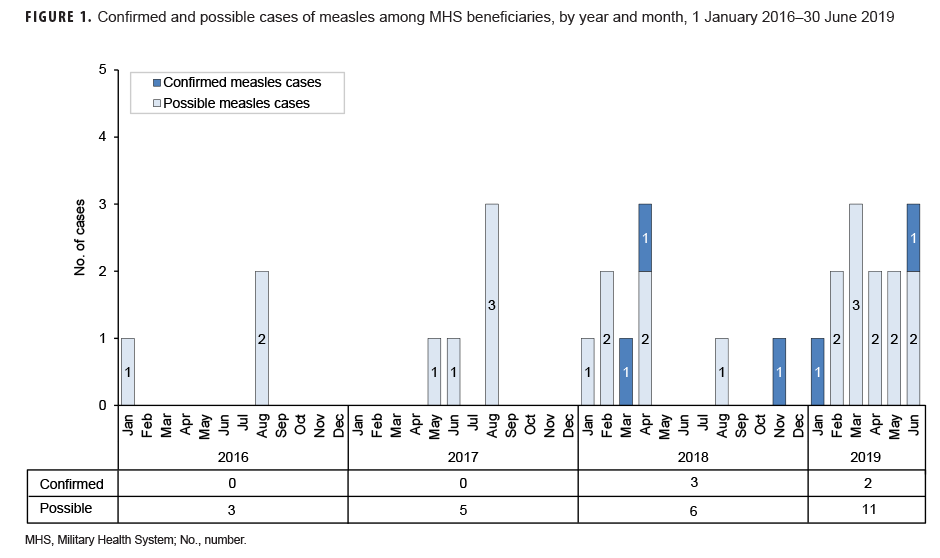
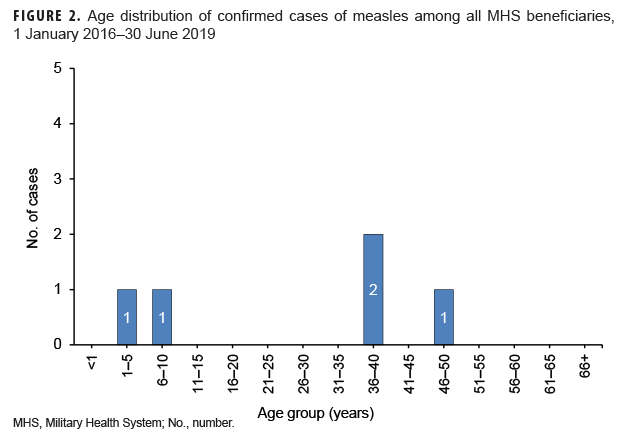
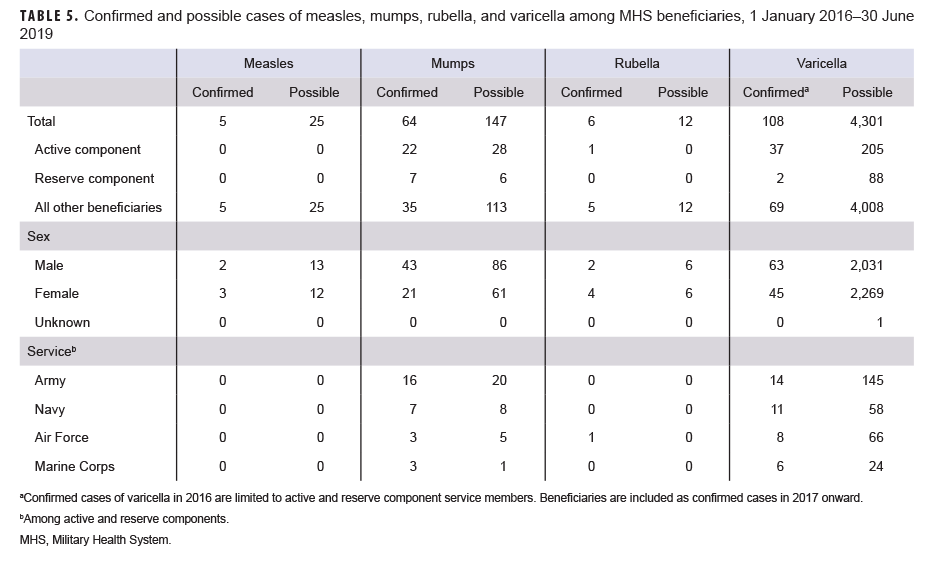
Measles: During the 3.5-year surveillance period, there were a total of 5 confirmed cases of measles among all MHS beneficiaries (Table 5, Figure 1). There were no confirmed cases of measles among service members; all 5 cases were among non-service member beneficiaries and 3 of those cases affected women (Table 5). Confirmed cases of measles were reported in 2018 (n=3) and during the first 6 months of 2019 (n=2) (Figure 1). Both of the cases in the first 6 months of 2019 were diagnosed in Texas (data not shown). Of the 5 confirmed measles cases reported during the surveillance period, 2 (40.0%) were among children 10 years old or younger (Figure 2); of these 2 children, 1 was 1 year old and 1 was 7 years old (data not shown). The remaining 3 cases were 37 years of age or older (Figure 2)
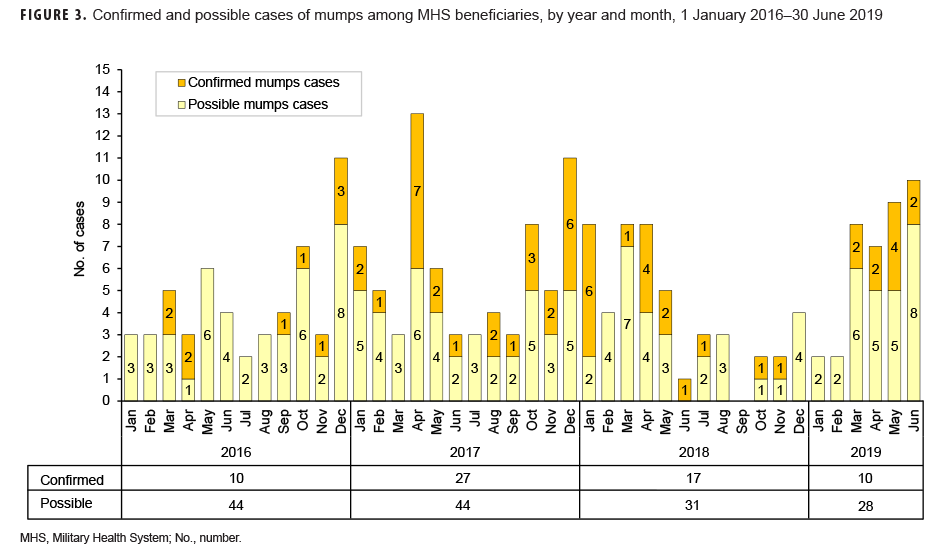
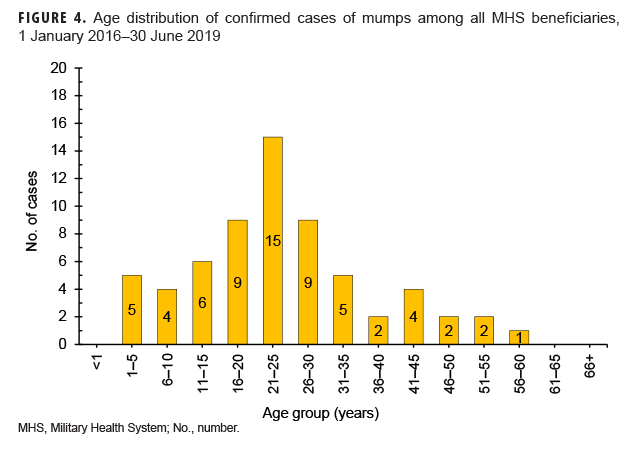
Mumps: There were 64 confirmed cases of mumps among all MHS beneficiaries during the surveillance period (Table 5, Figure 3). Slightly more than two-thirds (67.2%) of the confirmed mumps cases were among men. Twenty-two cases (34.4%) were among active component service members and 7 cases were among reserve component service members. Of the 29 confirmed mumps cases in service members, 16 cases were among Army members, 7 among Navy, 3 among Air Force, and 3 among Marine Corps members (Table 5). The remaining 35 confirmed mumps cases were among non-service member beneficiaries. During the surveillance period, the greatest number of confirmed cases was reported in 2017 (n=27) (Figure 3). There were 2 confirmed cases of mumps among service members in 2016, 17 in 2017, 5 in 2018, and 5 in the first 6 months of 2019 (data not shown). Overall, the single month with the highest number of confirmed mumps cases was April 2017 (n=7) (Figure 3). The 3 locations with the most confirmed mumps cases were Hawaii (n=13), Texas (n=12), and Alaska (n=6) (data not shown). The age group with the most confirmed cases was young adults 21–25 years old (n=15; 23.4%) (Figure 4).
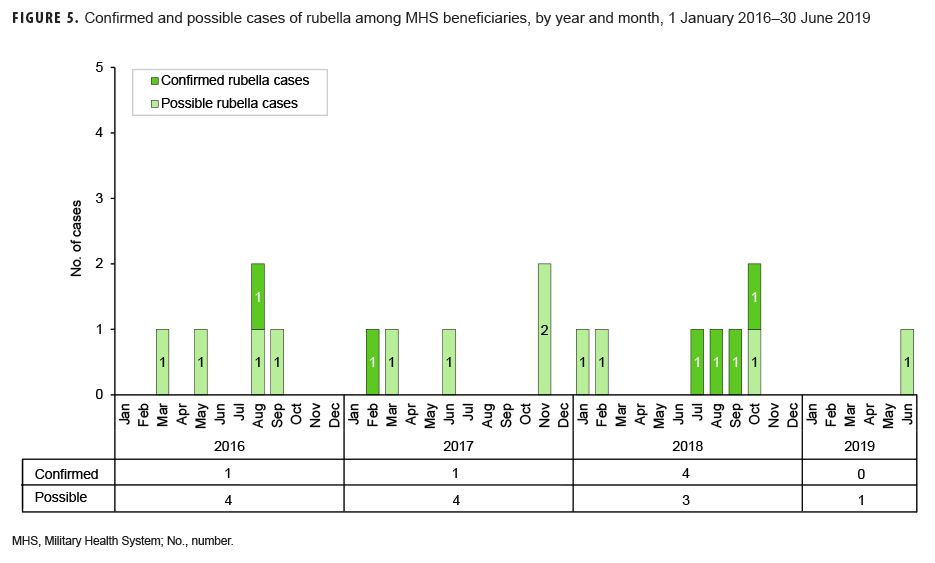
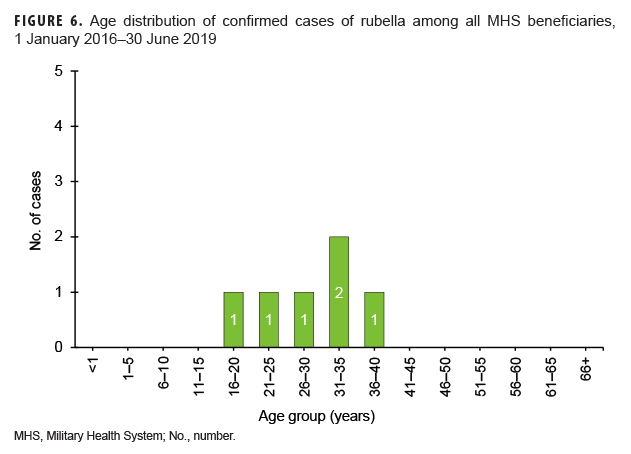
Rubella: During the surveillance period, there were 6 confirmed rubella cases among all MHS beneficiaries (Table 5, Figure 5). Two-thirds (66.7%) of confirmed rubella cases were among women. There was 1 confirmed case of rubella in an active component Air Force member diagnosed in October 2018 in Nebraska (data not shown). The remaining 5 confirmed rubella cases were among non-service member beneficiaries (Table 5). All 6 of the confirmed rubella cases were among adults 20–40 years old (Figure 6). Whereas 4 confirmed rubella cases were reported in 2018, only single confirmed cases of rubella were reported in 2016 and 2017 (Figure 5). No confirmed rubella cases were reported in the first 6 months of 2019.
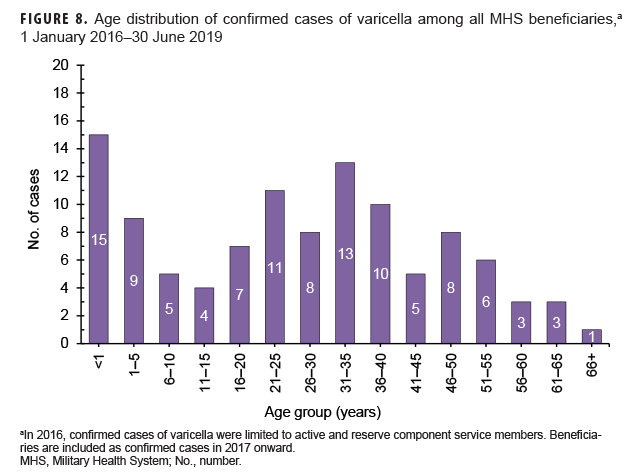
Varicella: There were 108 confirmed cases of varicella during the surveillance period (Table 5, Figure 7). Nearly three-fifths (58.3%) of confirmed varicella cases were among men. Thirty-nine (36.1%) of the confirmed cases of varicella were among service members. Of these 39 cases, 14 were among Army members, 11 among Navy members, 8 among Air Force, and 6 among Marine Corps members (Table 5). The vast majority (94.9%) of service members with confirmed varicella infections were active component members. There were 9 confirmed cases of varicella among service members in 2016, 11 in 2017, 11 in 2018, and 8 in the first 6 months of 2019 (data not shown). The time periods with the most confirmed varicella cases were 2018 (n=39) and the first 6 months of 2019 (n=37) (Figure 7). Overall, the months with the greatest number of confirmed varicella cases were Aug. 2018 (n=9) and April, May, and June 2019 (n=8, n=8, n=8, respectively) (Figure 7). The 3 locations with the most confirmed cases of varicella were Texas (n=22), Florida (n=13), and Virginia (n=10) (data not shown). The age groups with the most confirmed cases were infants less than 1 year old (n=15; 13.9%) and adults 31–35 years old (n=13; 12.0%) (Figure 8).
Possible cases
Measles: During the 3.5-year surveillance period, there were 25 possible cases of measles among all MHS beneficiaries (Table 5). None of the possible cases were among active or reserve component service members; all 25 of the possible cases were among non-service member beneficiaries. The number of possible measles cases reported in the first 6 months of 2019 (n=11) was more than 3 times the number reported in 2016 (n=3). The greatest number of possible measles cases was among children 5 years old or younger (n=18; 72.0%) (data not shown). Mumps: Overall, there were 147 possible cases of mumps among all MHS beneficiaries during the surveillance period (Table 5). Of these, 28 possible cases were among active component service members and 6 were among reserve component service members. The remaining 113 possible cases were among non-service member beneficiaries. The age groups with the greatest numbers of possible mumps cases were children aged 1–5 years old (n=23; 15.6%) and children aged 6–10 years old (n=22; 15.0%) (data not shown).
Rubella: During the surveillance period, there were 12 possible cases of rubella among all MHS beneficiaries (Table 5). All 12 possible cases of rubella were among non-service member beneficiaries. The greatest number of possible rubella cases was among children aged 1–5 years old (n=6; 50.0%) (data not shown).
Varicella: There were 4,301 possible cases of varicella during the surveillance period among all MHS beneficiaries (Table 5). Of these, 205 (4.8%) possible cases were among active component service members and 88 (2.0%) were among reserve component service members. The remaining 4,008 possible varicella cases were among non-service member beneficiaries. The age groups with the greatest numbers of possible cases of varicella were children aged 1–5 years old (n=1,338; 31.1%) and children 6–10 years old (n=579; 13.5%) (data not shown).
Editorial Comment
Current DOD policy is to screen the immunization records of accessions during initial entry training and immunize if the primary series against MMR/V is incomplete.29 Although DOD policy calls for serologic testing for antibodies to measles, rubella, and varicella (as well as hepatitis A and hepatitis B), current practice at military accession sites also includes mumps serology in accordance with CDC's Advisory Committee on Immunization Practices recommendations.29,32
Between 1 Jan. 2016 and 30 June 2019, no cases (confirmed or possible) of measles were reported among service members. All of the measles cases identified in this analysis were among non-service member beneficiaries. Children 10 years old or younger accounted for two-fifths of all confirmed measles cases during the surveillance period. This finding and those of published reports of recent outbreaks suggest that some children who have not received 2 doses of MMR or MMRV vaccine are susceptible to infection when exposed to the measles virus.30,31
During the 3.5-year surveillance period, there were more than 12 times as many confirmed cases of mumps (n=64) as there were of measles (n=5). This finding is not unexpected given that the efficacy of the mumps vaccine (88% [range: 66%–95%] with 2 doses; 78% [range: 49%–92%] with 1 dose) is lower than that of the measles component of the vaccine.32–34 It is also consistent with multiple studies showing that waning immunity may contribute to mumps outbreaks in settings where persons have close, prolonged contact.35,36 In the current analysis, the greatest number of confirmed cases of mumps occurred among 21- to 25-year-olds. Results of a recent synthesis of data from 6 mumps vaccine effectiveness studies suggest that vaccine-derived immune protection against mumps wanes on average 27 years (95% confidence interval: 16–51 years) after vaccination.5 This highlights the fact that increased outbreaks due to mumps are not fully explained and may be related to a combination of factors including waning immunity, vaccine escape mutations, and genetic differences in vaccine responsiveness.37,38
In the current analysis, Texas was the location associated with the greatest number of confirmed measles cases and the location associated with the second highest number of mumps cases among MHS beneficiaries. It is unknown whether these cases were associated with outbreaks within military or civilian communities.
The low number of confirmed rubella cases reported during the surveillance period is expected given the efficacy of the rubella component of the MMR vaccine and the low number of cases reported in the general U.S. population during this time.4
Across the services, the varicella vaccine is administered to susceptible trainees and other accessions within the first 2 weeks of initial entry training.29 Serologic screening is one means of determining susceptibility to varicella infection.18 Those individuals without a personal history of chickenpox, documentation of 2 prior varicella vaccinations, or documentation of immunity based on serologic testing are considered susceptible.29 Susceptible adults require 2 doses of varicella vaccine given 4–8 weeks apart.29 In 2017, the reporting policy for RMEs for varicella was changed to include all beneficiaries and is no longer restricted to only active and reserve component service members.24 The observed pattern of an increase in the numbers of confirmed varicella cases in 2017, 2018, and the first 6 months of 2019 relative to 2016 is likely due, at least in part, to this change in reporting policy.
As expected, this analysis identified many more possible cases of MMR/V than confirmed cases. One example of the challenges to complete ascertainment and counting of cases is provided by the recent, aforementioned outbreak of parotitis aboard the USS Fort McHenry in 2018. Although the final count of shipboard cases considered likely to be mumps was 28, only 23 cases (confirmed or possible) of mumps were reported for the entire DOD in this period. In the MHS, diagnoses of MMR/V require RME notifications. The published guidelines emphasize that the proper identification, treatment, control, and follow-up of cases requires prompt, accurate reporting of probable, suspected, or confirmed cases of these infections.24 In addition, the guidelines discourage delaying the submission of RME reports while awaiting laboratory confirmation and call for the submission of additional reports once the diagnosis has been confirmed.24 In the context of these guidelines, the current analysis searched the database of RMEs for cases that were identified as "confirmed." RMEs that characterized the diagnoses as either "probable" or "suspected" and were never amended as "confirmed" were treated as "possible" cases. Such cases were grouped with cases identified from records of inpatient and outpatient records. Consequently, "possible" cases may include both "true" cases for which there were no follow-up RMEs indicating confirmation and "true" cases for which diagnoses were documented in inpatient or outpatient records but no RMEs were ever submitted by local military public health officials. Because "possible" cases based upon diagnoses in the primary diagnostic position for inpatient or outpatient encounters required an additional diagnostic code for an associated symptom, some cases of true MMR/V infections are likely not captured as "possible" cases because documentation of a specific diagnosis was not accompanied by documentation of a symptom. This aspect of the case definition could lead to underestimation of total counts of "possible" cases. Civilian health care providers who diagnose and confirm cases of any of these 4 viral infections outside of the MHS would not be expected to submit RME reports; however, the diagnoses are captured in the DMSS if such care is underwritten by the MHS. Moreover, for 2017, 2018, and 2019, medical data from sites that were using MHS GENESIS, the new electronic health record for the MHS, are not available in the DMSS. These sites include Naval Hospital Oak Harbor, Naval Hospital Bremerton, Air Force Medical Services Fairchild, and Madigan Army Medical Center. Therefore, medical encounter data for individuals seeking care at any of these facilities during 2017–2019 were not included in the analysis. The scenarios and situations described above may result in the underestimation of the actual incidence of cases of MMR/V among MHS beneficiaries.
Conversely, other circumstances may tend to result in overestimation of the number of incident cases. For example, diagnoses of MMR/V recorded in electronic health records may represent misdiagnoses, tentative (rule-out) diagnoses that are not confirmed, and/or miscoding of medical encounters for vaccinations or laboratory testing. Because of this inherent uncertainty, counts of confirmed cases were the main focus of this report.
Recent trends in MMR/V in both military and civilian populations in the U.S. highlight the importance of primary and booster vaccinations. Current recommendations for the MMR vaccine include 2 doses—the first between 12 and 15 months and the second between 4 and 6 years old.39 Adults with only 1 dose or who lack laboratory evidence for MMR immunity are encouraged to receive the vaccine, particularly those who work in health care settings.39 Current recommendations for varicella vaccination correspond to the MMR vaccination schedule (2 doses—the first between ages 12 and 15 months and the second between ages 4 and 6 years), with a catch-up vaccination for susceptible children, adolescents, and adults.39 Because they are required to have evidence of immunity for MMR/V, it is not surprising that service members account for a relatively small proportion of all cases of these diseases in the MHS.
References
- Immunization Action Coalition. Vaccine timeline. http://www.immunize.org/timeline/. Accessed 29 Aug. 2019.
- Centers for Disease Control and Prevention. Atkinson W, Wolfe C, Hamborsky J. eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Washington, DC: Public Health Foundation; 2011.
- Patel M, Lee AD, Clemmons NS, et al. National update on measles cases and outbreaks—United States, Jan. 1–Oct. 1, 2019. MMWR Morb Mortal Wkly Rep. 68(40):893–896.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS). Notifiable infectious disease and conditions data tables. https://wwwn.cdc.gov/nndss/infectious-tables.html. Accessed 5 September 2019.
- Lewnard JA, Grad YH. Vaccine waning and mumps re-emergence in the United States. Sci Transl Med. 2018;10(433). pii:eaao5945.
-
6. Harling R, White JM, Ramsay ME, Macsween KF, van den Bosch C. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine. 2005;23(31):4070–4074.
- Clemmons N, Hickman C, Lee A, Marin M, Patel M. Chapter 9: Mumps. In: Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. 5th ed. Atlanta, GA: Centers for Disease Control and Prevention.
- Centers for Disease Control and Prevention. Mumps cases and outbreaks. https://www.cdc.gov/mumps/outbreaks.html. Accessed 21 Oct. 2019.
- Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358(15):1580–1589.
- Centers for Disease Control and Prevention. Elimination of rubella and congenital rubella syndrome—United States, 1969–2004. MMWR Morb Mortal Wkly Rep. 2005;54(11):279–282.
- World Health Organization. WHO vaccine preventable diseases: monitoring system. 2019 global summary. Incidence time series for United States of America (the) (USA). http://apps.who.int/immunization_monitoring/globalsummary/incidences?c=USA. Accessed 6 September 2019.
- Centers for Disease Control and Prevention. Rubella (German measles, three-day measles). https://www.cdc.gov/rubella/index.html. Accessed 6 September 2019.
- Lopez AS, Leung J, Marin M. Varicella outbreak surveillance in the United States, 2015–2016. Open Forum Infect Dis. 2017;4(suppl 1):s461.
- Centers for Disease Control and Prevention. Chickenpox (varicella). Varicella reporting and surveillance. https://www.cdc.gov/chickenpox/health-departments/conducting-surveillance.html. Accessed 9 September 2019.
- Centers for Disease Control and Prevention. Chickenpox (varicella). Monitoring the impact of varicella vaccination. https://www.cdc.gov/chickenpox/surveillance/monitoring-varicella.html. Accessed 9 Sept. 2019.
- Lopez AS, Kolasa MS, Seward JF. Status of school entry requirements for varicella vaccination and vaccination coverage 11 years after implementation of the varicella vaccination program. J Infect Dis. 2008;197(suppl 2):s76–s281.
- Lopez AS, Zhang J, Brown C, Bialek S. Varicella-related hospitalizations in the United States, 2000–2006: the 1-dose varicella vaccination era. Pediatrics. 2011;127(2):238–245.
- Marin M, Guris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1–40.
- Leung J, Harpaz R. Impact of the maturing varicella vaccination program on varicella and related outcomes in the United States: 1994–2012. J Pediatric Infect Dis Soc. 2016;5(4):395–402.
- Pickrell R. The crew of this Navy warship has gone without a port of call thanks to a viral mumps outbreak. Task & Purpose. 2 May 2019. https://taskandpurpose.com/uss-fort-mchenry-mumpsoutbreak. Accessed 6 September 2019.
- Montgomery N. Hundreds receive vaccinations after soldiers show signs of mumps in Italy. Stars and Stripes. 13 Aug. 2019. https://www.stripes.com/news/hundreds-receive-vaccinations-aftersoldiers-show-signs-of-mumps-in-italy-1.594165. Accessed 11 September 2019.
- Egnash M. Army stresses vaccinations after soldier treated for mumps in Germany. Stars and Stripes. 10 Aug. 2019. https://www.stripes.com/news/europe/army-stresses-vaccinations-aftersoldier-treated-for-mumps-in-germany-1.593891. Accessed 9 September 2019.
- Williams VF, Stahlman S, Fan M. Measles, mumps, rubella, and varicella among service members and other beneficiaries of the Military Health System, 2010–2016. MSMR. 2017;24(10):2–11.
- Armed Forces Health Surveillance Branch. Armed Forces Reportable Medical Events. Guidelines and Case Definitions, 2017. https://health.mil/Reference-Center/Publications/2017/07/17/Armed-Forces-Reportable-Medical-Events-Guidelines.
- Armed Forces Health Surveillance Branch. Surveillance Case Definitions: Measles. https://health.mil/Reference-Center/Publications/2015/09/01/Measles. Accessed 29 Aug. 2019.
- Armed Forces Health Surveillance Branch. Surveillance Case Definitions: Mumps. https://health.mil/Reference-Center/Publications/2015/05/01/Mumps. Accessed 29 Aug. 2019.
-
Armed Forces Health Surveillance Branch. Surveillance Case Definitions: Rubella. https://health.mil/Reference-Center/Publications/2018/01/01/Rubella. Accessed 29 Aug. 2019.
-
Armed Forces Health Surveillance Branch. Surveillance Case Definitions: Varicella. https://health.mil/Reference-Center/Publications/2018/01/01/Varicella. Accessed 29 Aug. 2019.
-
Headquarters, Departments of the Army, the Navy, the Air Force, and the Coast Guard. Army Regulation 40-563, BUMEDINST 6230.15B, AFI 48–110_IP, CG COMDTINST M6230.4G. Medical Services: Immunizations and Chemoprophylaxis for the Prevention of Infectious Diseases. 7 Nov. 2013.
-
Hall V, Banerjee E, Kenyon C, et al. Measles Outbreak–Minnesota April–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66(27):713–717.
-
Patel M, Lee AD, Redd SB, et al. Increase in Measles Cases–United States, Jan. 1–April 26, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(17):402–404.
-
McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS, Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-04):1–34.
-
Centers for Disease Control and Prevention. Mumps vaccination. https://www.cdc.gov/mumps/vaccination.html. Accessed 29 Aug. 2019.
-
Centers for Disease Control and Prevention. Measles Vaccination. https://www.cdc.gov/measles/vaccination.html. Accessed 29 Aug. 2019.
-
Hamami D, Cameron R, Pollock KG, Shankland C. Waning immunity is associated with periodic large outbreaks of mumps: a mathematical modeling study of Scottish data. Front Physiol. 2017;8:233.
-
Marshall HS, Plotkin S. The changing epidemiology of mumps in a high vaccination era. Lancet Infect Dis. 2019;19(2):188–119.
-
Zengel J, Phan SI, Pickar A, Xu P, He B. Immunogenicity of mumps virus vaccine candidates matching circulating genotypes in the United States and China. Vaccine. 2017;35:3988–3994.
-
Scepanovic P, Alanio C, Hammer C, et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018;10(59):1–13.
-
Centers for Disease Control and Prevention. Vaccine information statements (VISs). https://www.cdc.gov/vaccines/hcp/vis/current-vis.html. Accessed 28 Aug. 2019.