What Are the New Findings?
This report represents the first studied large outbreak of COVID-19 among U.S. MHS beneficiaries in Europe. Rapid control of cases was achieved with robust testing, case isolation, contact identification and quarantine of contacts. During the surveillance period, 7.1% of COVID-19 cases in the MHS beneficiary population and 4.4% of cases in the U.S. military population were hospitalized.
What Is the Impact on Readiness and Force Health Protection?
A better understanding of how COVID-19 affects the MHS population can assist MHS planners with preparing for COVID-19 in their communities.
Abstract
In 2020, the SARS-CoV-2 virus caused an unprecedented pandemic of coronavirus disease 2019 (COVID-19). Army Public Health Command Europe monitored all cases of COVID-19 for Military Health System (MHS) beneficiaries seen in Army Military Treatment Facilities. Cases entered into the Army Disease Reporting System internet (ARDSi) were evaluated for symptomatology as this was a younger and healthier cohort than typically reported on at the time. During the surveillance period, 7.1% of COVID-19 cases among MHS beneficiaries were hospitalized; these cases presented with symptoms such as congestion, sore throat, and disturbances in taste and smell. Interventions to stop the outbreak included aggressive case finding with robust testing, control of cases and contacts, and the extreme social distancing measures seen in other countries. The outbreak was successfully brought under control in one month. Cases remained sporadic and were due largely to importation from the U.S. until the end of Aug. 2020.
Background
As of 19 April 2020, the World Health Organization (WHO) reported over 2 million cases of COVID-19 causing over 148,000 deaths in 213 countries.1 As of 18 April 2020, 137,000 cases and over 4,000 deaths were reported in Germany.2 As of 17 April 2020, 2,986 cases were reported among Military Health System (MHS) beneficiaries.3 U.S. military personnel, their families, government contractors, and general schedule (GS) employees undergo medical screening prior to overseas assignments to ensure medical needs can be met at the assigned duty station.4 Because of this screening, some patients with chronic diseases requiring specialized medical care may be prevented from transferring overseas leading to a healthier cohort than the average U.S. population.3,5
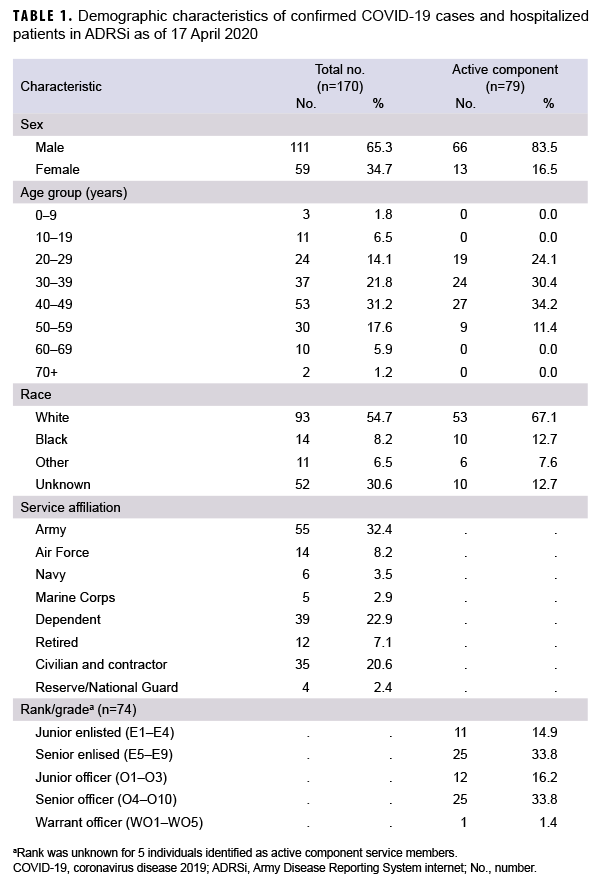
The MHS uses a medical event reporting system known as Disease Reporting System Internet (DRSi). This is a service specific system and the Army's version of the system is known as ADRSi. Cases of COVID-19 are reported to DRSi based on the service affiliation of the medical facility at which the patient was seen, not based on the service affiliation of the patient. A confirmed case of COVID-19 was a patient with evidence of SARS-CoV-2 in a clinical specimen using a molecular amplification test, regardless of symptoms.5 The Army has multiple medical facilities throughout Europe; however, the greatest concentration is in Germany. Locations of Army medical facilities can be found on the cover page of Regional Health Command Europe's website.6 U.S. Army Public Health Command Europe (PHCE), which monitors ADRSi for Army Facilities throughout Europe, recorded its first confirmed case of COVID-19 on 12 March 2020. Through 17 April 2020, 170 patients were identified as confirmed cases based on having met laboratory criteria for SARS-CoV-2 infection. An additional 73 cases among MHS beneficiaries not reported through the ADRSi were noted throughout Europe from the beginning of the pandemic through April 17th. The majority of the remaining cases were reported in the United Kingdom and Italy. This report describes the epidemiology of the 170 cases of COVID-19 reported in ADRSi from 12 March 2020 through 17 April 2020.
Methods
A database of confirmed cases was developed from the ADRSi entries reported to Army PHCE from the date of the first case identified (2 March 2020) through the end of the first wave of cases (17 April 2020). Information on symptoms, past medical history, and demographics was obtained from the Armed Forces Health Longitudinal Technology Application (AHLTA) and Health Artifact and Image Management Solution (HAIMS) and added to the study database. Laboratory reports in AHLTA were utilized to verify that patients listed in the ADRSi were confirmed COVID-19 cases. Individuals presenting to medical facilities with symptoms were directed to COVID-19 screening locations where preprinted symptom questionnaires were used. These questionnaires were not standardized across the MHS, but most U.S. Army medical facilities in Europe used 1 or more of 3 different questionnaires. The most basic questionnaire that was used most frequently in medical encounters included prompts for symptoms of cough, fever, headache, and myalgias. Two more complex questionnaires included most other symptoms except loss of taste and loss of smell. Patients were more frequently asked about loss of taste and smell after 23 March when medical personnel in the region became aware that this was a common symptom complex. Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA) was used to tabulate cases.
Results
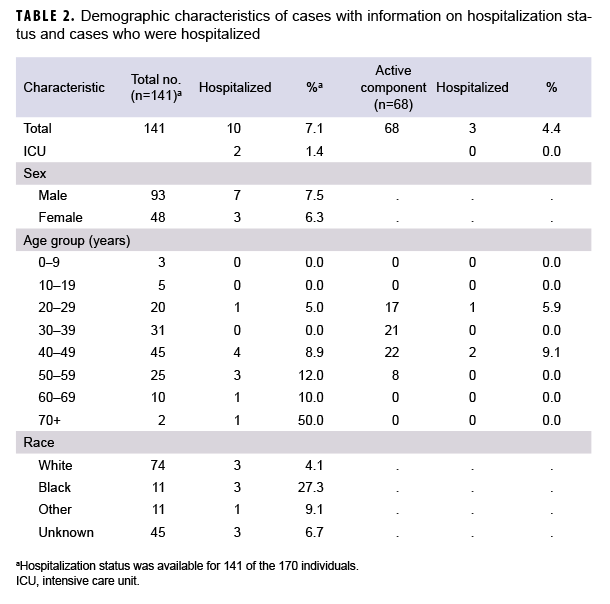
Of the 170 confirmed COVID-19 cases in the study database, 163 (95.9%) were diagnosed at medical facilities in Germany. Twenty-nine patients (17.1%) had a date of symptom onset listed, but specific symptoms and hospitalization status were not available in the ADRSi, AHLTA, or HAIMS. Six (4.3%) of the 141 confirmed cases with symptom information were asymptomatic. Of the 141 cases with symptom information, 68 (48.2%) were identified as active component service members (ACSMs). The age range of ACSM cases was 20 to 56 years. Non-ACSM cases ranged in age from 2 to 72 years with a median age of 41 years (interquartile range=31–49 years). Males made up 65.3% of all cases, 83.5% of ACSMs, and 49.5% of non-ACSMs. Whites accounted for 54.7% of all cases, 67.1% of ACSMs, and 44.0% of non-ACSMs. Blacks accounted for 8.2% of all cases, 12.7% of ACSMs and 4.4% of non-ACSMs (Table 1). Those of unknown or other race accounted for the remainder of cases. The data sources used did not specify ethnicity. A total of 10 (7.1%) patients were hospitalized, and of these, 2 required intensive care (Table 2). All patients were discharged and there were no deaths among the cases during the study period.
Hypertension was prevalent in the past medical histories of 14.1%, of cases, tobacco use history in 12.9%, diabetes in 4.1%, asthma in 4.1%, and cardiovascular disease in 1.8% (data not shown). For hospitalized patients (n=10), hypertension was prevalent in the past medical histories of 20.0% of cases, diabetes in 10.0% and cardiovascular disease in 10.0% (data not shown). Of the 2 hospitalized cases who required intensive care, both had at least 2 medical conditions (data not shown). Obesity, as determined by a diagnosis in the medical record, was present in 1.8% of all cases and 10.0% of hospitalized patients. No significant past medical history was found in 54.7% of all cases and 30.0% of hospitalized cases (data not shown).
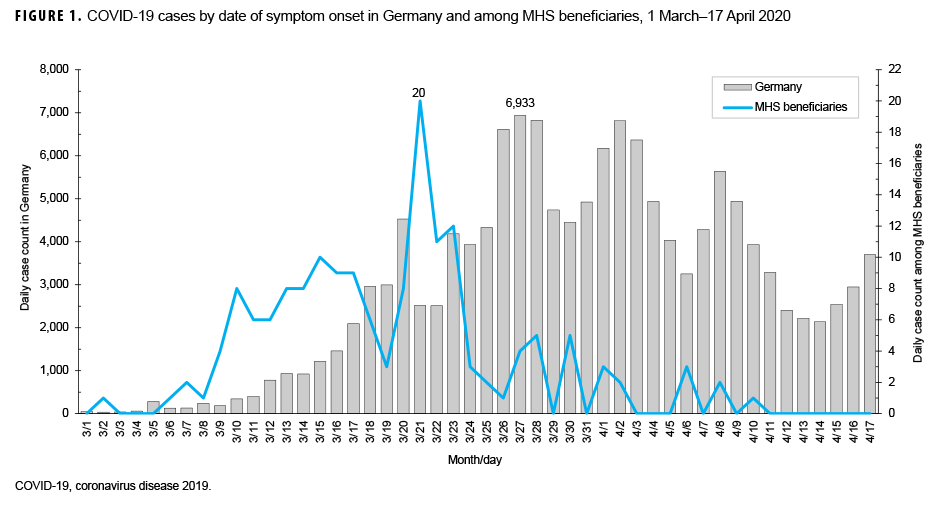
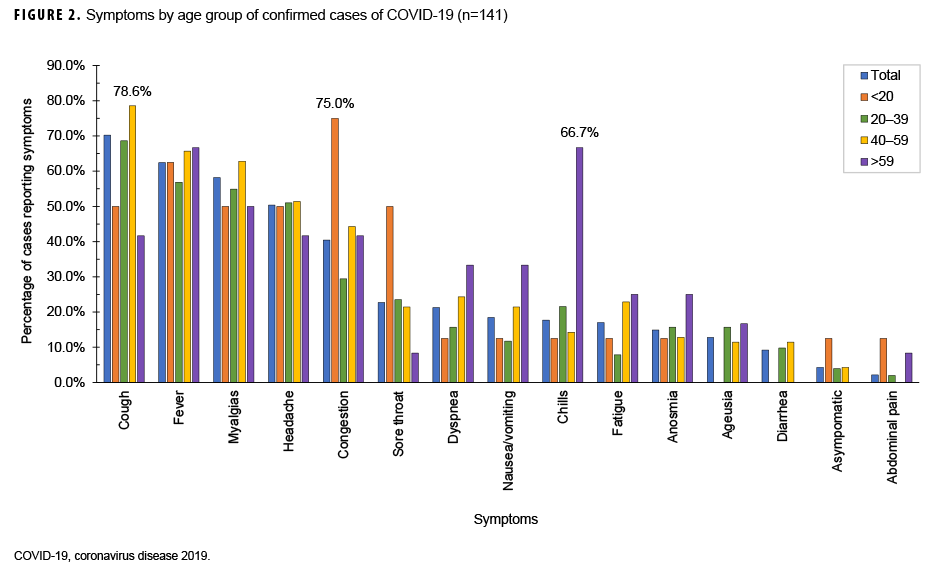
Date of symptom onset was known for each of the 164 symptomatic patients. The epidemic curve, with respect to symptom onset, is presented in Figure 1. Confirmed cases began 2 March, peaked 21 March, and were sporadic from 1 April through the end of August 2020. The most common symptoms reported were cough (70.2%), fever (62.4%), myalgias (58.2%), and headache (50.4%) (Figure 2). When symptoms were stratified by age group, the main deviations from the norm were that congestion (75.0%) was the most common symptom in individuals under 20 years of age and chills (66.7%) were the most common symptom in individuals over 59 years of age. Anosmia (loss of smell) was reported in 14.9% of cases. A total of 84.4% of cases had cough or fever as symptoms and 88.7% of cases had cough, fever, myalgias, or headache as symptom (data not shown).
Editorial Comment
The most common symptoms reported in this population were cough, fever, myalgias, and headache. In this population, stratification by age group resulted in small cell sizes, but there was an indication that younger people did have more mild symptoms such as congestion whereas older people were more likely to present with symptoms that could lead to hospitalization, such as dyspnea.
On the day the first case of COVID-19 was reported to Army PHCE, Germany had approximately 3,000 cumulative cases.2 Germany saw a rapid increase in cases over the course of the next week and more strict suppression measures went into effect such as closing schools, requiring 1.5 to 2 meter distance in all in-person transactions, and not allowing more than 2 unrelated people to be together.7 On 26 March, bases throughout Germany instituted policies of only allowing personnel to travel from home to work or other essential locations such as grocery stores and medical facilities. Additionally, in alignment with Germany's aggressive approach to evaluating cases and contacts, military public health personnel conducted thorough case finding with notifications on social media about "hot spot" locations and testing of potential cases, contacts, and those who may have been exposed, regardless of the severity of symptoms. The rapidly instituted measures of robust case finding, testing, and "hot spot" notifications in conjunction with social distancing led to a significant decrease in the daily count of cases among U.S. military personnel and their families in Europe.
Aside from retirees, all other personnel in the dataset are subject to some form of medical screening prior to an overseas assignment. This makes the population less generalizable to the U.S. population. Additionally, 46.5% of all confirmed cases in this data set were active component military, increasing the "healthy worker" effect. Symptom data were not available for 17.1% of cases. It is reassuring that, based on thorough record review, these additional cases were not hospitalized; however, it is unclear what symptoms they may have experienced. This is also likely an undercount of total cases in Germany. Prior to 21 March 2020 guidelines for testing only those with either fever or cough and known contact with an infected individual or travel were adhered to. Additionally, the known false negative risk of RT-PCR8 likely led to fewer cases being captured than actually existed. A clinical case definition was released on 24 March; however, only laboratory confirmed cases were included in this dataset. Low counts of hospitalized cases precluded generalizations about such patients.
Lessons can be gleaned from the U.S. military experience with COVID-19 in Europe. In alignment with the WHO's COVID-19 Strategy Update of 14 April 2020,9 control of COVID-19 is very dependent on absolute control over cases. To achieve this level of control, cases must be identified with testing, regardless of how limited their symptoms may be. Cases must be isolated and their contacts must be identified and quarantined. Due to the risk of asymptomatic spread, all individuals must be vigilant about maintaining social distance and recognizing possible symptoms in themselves and others. These aggressive strategies have significantly decreased cases in a rapid fashion in U.S. military personnel in Europe and can be employed in other military settings to gain control over COVID-19.
Author affiliation: Navy Environmental and Preventive Medicine Unit SEVEN, U.S. Navy (CDR Servies).
Acknowledgements: Mahendra Kabbur, DVM, PhD, and Rodney Coldren, MD, MPH of U.S. Army Public Health Command Europe provided the ADRSi dataset and have been leading the charge against COVID-19 in Europe.
Disclaimer: The contents, views, or opinions expressed in this publication are those of the author and do not necessarily reflect official policy or position of the U.S. Army, the U.S. Navy, or the Department of Defense. The author is a military service member and employee of the U.S. Government. This work was prepared as part of her official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C., §101 defines a U.S. Government work as a work prepared by a military Service member or employee of the U.S. Government as part of that person's official duties.
References
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Published 2020. Updated April 19, 2020. Accessed April 19, 2020.
- Robert Koch Institute. Coronavirus Disease 2019 (COVID-19) Daily Situation Report of the Robert Koch Institute. Robert Koch Institute. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-04-18-en.pdf?__blob=publicationFile. Updated 18 April 2020. Accessed 19 April 2020.
- Armed Forces Health Surveillance Division. Coronavirus Disease 2019 (COVID-19) Outbreak Update #39. In. Silver Spring MD: Armed Forces Health Surveillance Division; 2020.
- US Army Medical Department. Screening for Overseas Assignments. U.S. Army Medical Command. https://efmp.amedd.army.mil/screening/assignments.html. Published 2010. Updated 31 Aug. 2010. Accessed 19 April 2020.
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) 2020 Interim Case Definition, Approved April 5, 2020. Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/. Updated 5 April 2020. Accessed 1 Dec. 2020.
- Regional Health Command Europe. Army Medicine Europe. https://rhce.amedd.army.mil/index. cfm. Updated 9 Dec. 2020. Accessed 9 Dec. 2020.
- Stafford N. Covid-19: Why Germany's case fatality rate seems so low. BMJ. 2020;369:m1395.
- Xiao AT, Tong YX, Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J Med Virol. 2020.
- World Health Organization. COVID-19 Strategy Update. Geneva, Switzerland: World Health Organization; 14 April 2020.