Department of Defense Directive (DODD) 6490.02E stipulates that health surveillance is essential to the evaluation, planning, and implementation of public health practice.1 A reliable system for capturing and communicating the occurrence of reportable medical events (RMEs) is a critical component of health surveillance within the DOD. Since its implementation in 2010, the Disease Reporting System internet (DRSi) has improved the timeliness of reporting and disease capture rates of RME surveillance for the DOD. However, numerous surveillance gaps still remain, in part, because many military treatment facilities (MTFs) lack written procedures for ensuring disease capture or DRSi utilization. Furthermore, the public health personnel of many MTFs need more knowledge of and training on the proper utilization of the RME case definitions and the submission of complete reports through the DRSi. These shortcomings limit the sensitivity and specificity of the passive surveillance system. While these gaps are not the only constraints identified by DRSi users, each significantly impacts surveillance for the DOD overall. This article will attempt to describe some of the deficiencies observed by experienced users of the DRSi and will elaborate on some tools developed by the Army Public Health Center (APHC) to improve RME surveillance, including a communicable disease toolkit.
Current practices for RMEs
Before 2010, the RME system used by the Army was only available for 35 locations. In an effort to improve surveillance of RMEs in the Army population, U.S. Army Medical Command (MEDCOM) adopted the Navy's DRSi in 2010.2 The internet-based interface allows more users and installations, including those located outside the continental U.S., to access the system. In addition, data submitted to the system are immediately available for review and analysis.
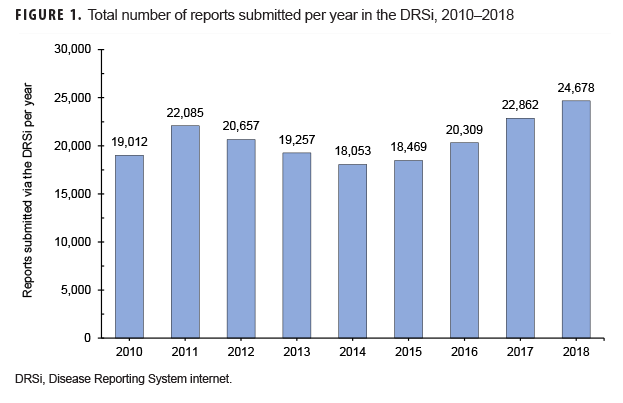
Overall usage of the DRSi in the Army increased by 30% from 2010 through 2018 in terms of the number of reports entered into the system (Figure 1).3 The most commonly reported RMEs from Army MTFs during this period were sexually transmitted infections (STIs), with STI reports accounting for 83% of all RMEs. Heat- and cold-related illnesses accounted for 5% of all reports, and gastrointestinal RMEs accounted for approximately 4% of all reports (APHC, unpublished data, 2019).
Cases may be entered into the DRSi by public health professionals at the local MTF on a daily basis, but the responsibilities of RME surveillance encompass several positions at the MTF. To start, the local public health professional should verify the patient's diagnosis using laboratory records and electronic medical records and then report that case into the DRSi within 2 business days of the diagnosis.3,4 If confirmatory laboratory data are still pending, the case must be reported as a preliminary case but then updated as new information becomes available. All required data elements outlined in the RME guidelines must be included in the medical event report in the DRSi.5 The case classification (i.e., suspected, probable, or confirmed) selected for that medical event report should conform to the current version of the Armed Forces RME Guidelines and Case Definitions.5
Cases reported to the DRSi from Army MTFs are reviewed and verified by epidemiologists at the APHC for accuracy and completeness on a daily basis. Information entered into the medical event report is checked against the RME guidelines. If the information meets the required criteria, the case is accepted without revisions. If necessary information is missing or the case classification does not match the information entered, the APHC contacts the original recorder of that report for clarification and additional information. This quality control measure ensures that the medical event reports entered in the DRSi database are reliable and standardized across all Army MTFs. The details of this secondary review process currently vary among the services, thus diminishing the validity of service-to-service comparisons when examining RME trends.
In their surveillance of notifiable diseases, the Centers for Disease Control and Prevention (CDC) and state and local health departments face challenges similar to those faced by the DOD.6–9 RME reporting in the civilian public health sector is based on passive surveillance through internet-based surveillance systems. Data on RMEs are captured through emergency room logs, laboratory results, infection control practitioners, and/or astute physicians or nurses at local clinics and are reported through the National Notifiable Disease Surveillance System. However, unlike the model used in the DOD, civilian public health professionals are required to document the disease follow-up for each RME.10 Standardized reporting and case investigation forms created and disseminated by the state or local health department are used to guide the investigations. Military installations are required to report notifiable conditions to the local and state health departments in parallel with DOD reporting through the DRSi. Since standardized investigation forms and processes for follow-up specific to the military are not available, investigations by DOD public health entities differ between installations, as they do between states.
Case follow-up is a critical step in surveillance of RMEs and includes activities such as verification of the diagnosis, determination of whether other individuals may be at risk of the same condition, post-exposure screening and prophylaxis, and other actions that protect and assure public health. Case follow-up augments a purely passive surveillance system with an active primary prevention component. In the DOD, thorough case follow-up occurs most consistently for STIs, as when public health nurses seek to identify and notify sexual contacts about exposure to a patient with an STI.11 This contact tracing is tracked through risk surveys, which are available through the DRSi and linked to each STI medical event report. Standardized questions on sexual behaviors are asked of each STI case. In the Army, the percentage of total STI reports with completed risk surveys is tracked quarterly for each installation and region. For example, Army MTFs in the Regional Health Command-Atlantic region completed risk surveys for 87% of all chlamydia cases reported from Oct. through Dec. 2018.11
Limitations to the current practice across the Army
The known gaps in RME follow-up are multifaceted and not isolated to 1 discrete cause. The gaps discussed in the current article were discovered through discussions with Army Preventive Medicine Chiefs, Army Public Health Nurses, and surveys of Army DRSi users carried out in 2017 and 2018. Challenges in RME follow-up unique to Air Force and Navy installations are not included in the current article. It should be noted that individuals whose public health duties at Army installations include the submission of RME reports through the DRSi are referred to here as "reporters."
Problems at the local level
The DOD struggles with reliable and consistent disease follow-up because of a lack of policy or command emphasis. Current DOD and Army regulations mandate reporting of specific RMEs, yet these same policies do not mandate public health surveillance practices for disease follow-up. However, DODD 6490.02E does state that the services are to implement early intervention and control strategies using practices consistent across the DOD.1 As a result, although public health officials and leadership may be made aware of the occurrence of individual cases of specific conditions, there may be no supplemental evidence to suggest that cases are related or that other persons may be at risk. For example, because the performance of contact tracing and the collection and analysis of food histories for non-STI RMEs are not methodically tracked by higher headquarters, it is not known whether or not such surveillance actions are consistently performed at Army MTFs. The only indication that food histories were taken for potential foodborne illnesses may be information in the comments section of the medical event report in the DRSi, but evidence within DRSi reports suggests that such interviews are often incomplete or not performed with scientific rigor. For example, 1 comment associated with a culture-confirmed case of campylobacteriosis stated "consumed leftover turkey legs [and] 4–5 hours later he started to have symptoms."12 Given the incubation period of 2–5 days for campylobacteriosis, this meal consumed by the patient is not likely to be the source of infection; however, no other information was provided regarding the possible source.
Frequent turnover in the staff who function as DRSi reporters commonly results in the placement of new reporters who need detailed training in the necessary knowledge base and procedures involved in RME reporting. A lack of training for new DRSi reporters is a significant problem that may be partially due to frequent staff turnover. A 2018 survey of Army medical event reporters completed by Battelle found that less than 50% of DRSi-using survey respondents had ever received any DRSi training. Further, approximately 60% of the DRSi-using respondents reported that their initial training for the system was learned on the job, and only 20% reported receiving training from a mentor. Frequent turnover in military health systems is inevitable; however, a lack of standard operating procedures (SOPs) that include guidelines for training exacerbates the negative impact of turnover on reporting compliance and data quality.
Effective, continuous surveillance based upon RME data depends upon DRSi reporters identifying cases of reportable conditions from notifications by health care providers or through regular searches of diagnoses recorded in patient records or laboratory results. A recent study by the APHC found that the percentage of diagnoses qualifying as RMEs that were reported as such through the DRSi (i.e., case capture percentage) ranged from 65–95% (mean=91%) (Army Institute of Public Health, unpublished data, 2013). Even a case capture percentage of 90% represents a concerning reduction in the sensitivity of the surveillance system. Qualitative assessment of this gap has found that case capture is sometimes difficult at the MTF level because local public health personnel lack the knowledge and abilities required to access medical systems of information. The APHC may be unaware of these training gaps unless substantial differences between laboratory and DRSi data are seen. Without evidence of such discrepancies, the APHC relies on reporters and their public health leadership at the local level to identify and communicate case reporting challenges.
Funding and established reporting responsibilities at each MTF vary significantly across the DOD. Some MTFs employ a team of reporters whose sole responsibility is to report diseases, while others assign 1 individual to handle reporting in addition to their other occupational responsibilities. Army regulation requires that all medical events on the current RME list be reported into the DRSi as soon as possible after the diagnosis has been made but no later than 2 business days from the diagnosis date.3,4 This standard includes case reports from subordinate and satellite clinics. In addition, local and state health departments have their own requirements for RME reporting timeliness. Reporting RMEs to multiple authorities with differing requirements and within a specified timeframe poses a significant burden to disease reporters. This burden becomes more substantial with fewer available reporters and can lead to a delay in the timeliness of reporting associated with the entire MTF. In addition, as military installations send more patients to civilian MTFs, barriers to communication may develop between civilian health agencies and military public health personnel.
Disease reporting as a challenging job duty
DRSi reporters have described difficulties interpreting specific case criteria and classifications for RMEs as presented in the RME guidelines. Some diseases have simple, laboratory-based definitions (e.g., "positive culture from any clinical specimen") and others are more complex, requiring interpretation of multiple laboratory results (e.g., "at least a 4-fold increase of antibody titer between acute and convalescent sera separated by 6–8 weeks
).5 Sickbert-Bennett et al. found that, in civilian public sector surveillance systems, diseases with fewer clinical criteria and laboratory-based case definitions tend to have higher completeness of reporting.9 This tendency underestimates the true burden of diseases with more clinical criteria and no laboratory-based case definitions. A similar gap likely exists within the DOD surveillance system; however, no studies comparing the DOD and civilian systems have been performed.
Historically, outbreak reporting in the Army has been inadequate because of a lack of clear understanding of what constitutes an outbreak. Moreover, what information to include in an RME outbreak report varies considerably between reporters. After action reports from outbreaks such as the Legionella outbreak in Selfridge, MI, and the atypical pneumonia outbreak at Fort Leonard Wood specifically noted this gap.13–15 Often, these reports contain insufficient information to answer basic public health-related questions. Since 2010, 104 outbreak reports have been submitted to the DRSi from 30 Army MTFs. Of the 104 outbreak reports, 30 (29%) did not specify the number of laboratory-confirmed cases and 60 (58%) were incomplete.16 Annual training has been provided by the services on how to identify outbreaks, strategies for investigating outbreaks, and how to report outbreaks in the DRSi; however, not all outbreaks are being reported and the APHC often learns of these outbreaks from situational reports, media, or personal contacts throughout the DOD.16
In summary, the gaps in RME-based surveillance described above pertain to the need for 1) detailed follow-up investigation of cases to identify contacts and possible risk to others; 2) training of public health investigators and DRSi reporters to allow more efficient execution of their duties; 3) improvement in the completeness of identification of cases that warrant DRSi reporting; and 4) enhancements in the recognition and investigation of outbreaks and the quality of outbreak reports. In order to reduce these gaps found in disease surveillance across the DOD, the following strategies for improving RME surveillance are recommended.
Tools needed to improve RME surveillance
Fundamental guidelines for reporting
Each DOD public health professional must be very familiar with the current list of conditions required to be reported by DOD policy and by their state and/or local public health authorities.5 Each MTF's public health team should ensure that lists of the reportable conditions are on display throughout the MTF so that health care providers who make diagnoses of such conditions are continuously reminded of the need to notify public health professionals when appropriate. The effort to display these lists throughout the MTF requires initiative and planning on the part of leadership and the staff. The current DOD list includes 68 RMEs, some of which are reportable only by DOD policy and not by state laws. The lists should be clearly visible throughout the MTF and regularly reviewed and updated when changes are made to the RME guidelines.
A copy of the RME guidelines5 must be available to all staff responsible for routine RME reporting. Each RME has specific clinical, laboratory, and exposure criteria that constitute a case definition. Although the case definitions were intended to correspond with those written by the CDC, there are some important differences. Therefore, it is crucial that staff review RME case definitions while entering cases into the DRSi to ensure they are completely and accurately reporting case information. Failure to refer to the case definition will result in incorrect medical event reports and a delay in timely notification of RMEs.
Having SOPs for the public health/preventive medicine staffs of the MTFs is an important option to promote improved reporting and surveillance of RMEs. SOPs offer a method to standardize case capture procedures and to provide installations with consistency over time. Staff turnover, lack of resources, and change of leadership all contribute to a work environment that is not conducive to accurate and reliable data reporting. These SOPs should provide location-specific guidance and outline how to perform RME follow-up, train new staff to ensure consistent disease surveillance, and conduct an outbreak investigation. For a more detailed example, readers are advised to see the epidemiological training session provided by the services in Jan. 2019 that discussed the importance of implementing an SOP and provided templates for MTFs to develop their own.17
Communicable disease toolkit
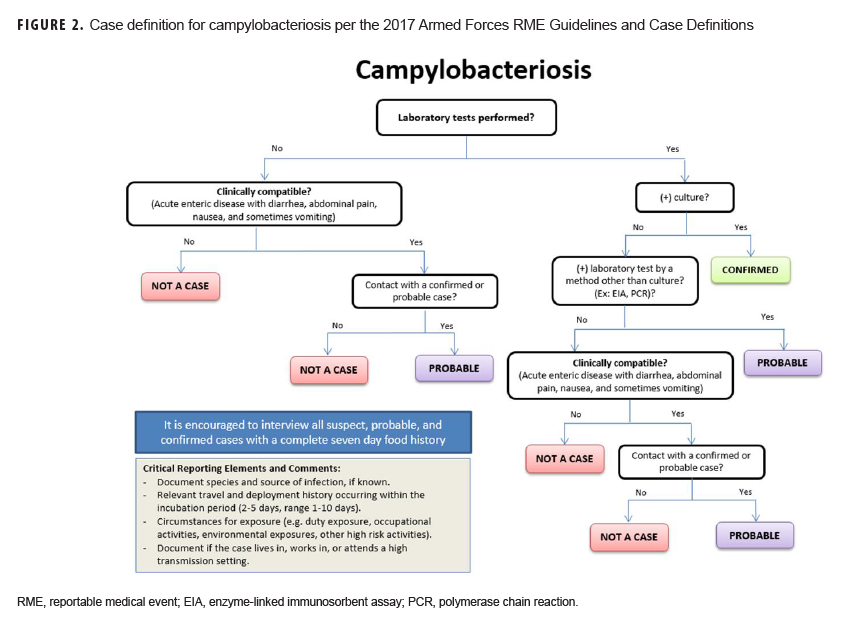
To help standardize the investigation process in response to outbreaks and RME surveillance in the DOD, a communicable disease toolkit was developed, and a first draft is currently undergoing technical and editorial review. The initial release will include factsheets, flow charts of the case definition (Figure 2), and standardized investigation forms for every RME. Future iterations of the toolkit will include template SOPs with guidance for documenting case findings, conducting outbreak investigations, managing disease follow-up, and carrying out epidemiologic analyses. Electronic copies of the toolkit will be available for download and will serve as a standardized resource for all MTFs to aid in disease reporting and surveillance according to DOD policy.
Once the communicable disease toolkit is available, significant marketing and training for all DOD MTFs will be required to encourage the actual use of the resources available to avoid the creation of MTF-specific toolkits. The aforementioned survey found that less than 55% of respondents used case definitions while entering cases in the DRSi. Furthermore, only 45% said that they referenced the RME guidelines for medical event reporting. It is unknown what guidelines the remaining 55% are using while reporting RMEs. These observations highlight the possibility that MTFs are implementing their own policies or lack an understanding of RME reporting. Training and marketing to MTFs to use the toolkit as well as the RME guidelines in their follow-up and disease reporting in the DRSi must be an ongoing process to ensure that new and existing staff are trained and aware of this important resource. In addition, DOD and service policy changes to mandate RME follow-up to improve surveillance and early outbreak detection should be considered.
Recommendations
Army RME practices present several opportunities for improvement. With ongoing support from leadership, implementing the recommendations of this proposal will result in improved RME surveillance, which will then result in improved public health response and thus increased force health protection and readiness. Adherence to case definitions results in data consistency, which then allows for meaningful comparisons and analyses for future studies. Questions such as, "Are these cases related?" cannot be reliably or consistently answered in the current system without significant additional investigation by the service public health centers with support from the MTFs.
The communicable disease toolkit contains resources that both simplify and standardize the reporting process across the DOD. The simplification of the reporting process will reduce the time burden on our public health professionals and encourage quality improvement on overall public health tasks. Outbreak identification would similarly be improved, which could enable a faster public health response. Similar toolkits have been made available by state and local public health departments, but a standardized resource for the DOD MTFs has been lacking for many years.
There are several opportunities to enhance disease reporting in the DOD, and ideally the recommendations discussed in this article can translate to other disciplines within the DOD public health realm. The end result may be a more efficient surveillance system for assessing the health status of the population it serves. Use of this system will result in clearer understanding, communication, and education on the importance of RME surveillance. Addressing the surveillance gaps discussed in this paper is an important first step to establish the foundation for public health intervention and impact. Additional interventions to improve surveillance include periodic surveillance evaluation studies, informatics solutions, new policies, better integration with CDC and civilian surveillance systems, and increased accountability measures.
Author affiliations: Defense Health Agency (Dr. Ambrose, Ms. Kebisek, Dr. White, Dr. O Donnell); Army Public Health Center, Aberdeen Proving Ground, MD (Mr. Gibson)
References
- Headquarters, U.S. Department of Defense. Directive 6490.02E. Comprehensive Health Surveillance. 2017.
- Headquarters, U.S. Army Medical Command. Operation Order 10-78 (Disease Reporting System-Internet). Sept. 2010.
- Headquarters, Department of the Army. Army Regulation 40-5 (Preventive Medicine). 25 May 2007.
- Headquarters, Department of the Army. Pamphlet 40-11 (Medical Services, Preventive Medicine). 19 Oct. 2009.
- Defense Health Agency. Armed Forces Health Surveillance Branch. Armed Forces Reportable Medical Events Guidelines and Case Definitions, 2017. https://health.mil/reference-Center/Publications/2017/07/17/Armed-Forces-Reportable-Medical-Events-Guidelines. Accessed 28 Jan. 2019.
- Council of State and Territorial Epidemiologists. Review of and recommendations for the National Notifiable Disease Surveillance System: a state and local health department perspective. https://cdn.ymaws.com/www.cste.org/resource/resmgr/PDFs/NNDSS_Report.pdf. Published April 2013. Accessed 28 Jan. 2019.
- Richards CL, Iademarco MF, Anderson TC. A new strategy for public health surveillance at CDC: improving national surveillance activities and outcomes. Public Health Rep. 2014; 129(6):472–476.
- Vest JR, Caine V, Harris LE, Watson DP, Menachemi N, Halverson P. Fostering local health department and health system collaboration through case conferences for at-risk and vulnerable population. Am J Public Health. 2018;108(5):649–651.
- Sickbert-Bennett EE, Weber DJ, Poole C, MacDonald PDM, Maillard J. Completeness of communicable disease reporting, North Carolina, USA, 1995–1997 and 2000–2006. Emerg Infect Dis. 2011;17(1):23–29.
- Adams D, Fullerton K, Jajosky R, et al. Summary of notifiable infectious diseases and conditions—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62(53):1–122.
- U.S. Army. Public Health Management System. Metric—Gonorrhea and Chlamydia Cases with Contact Tracing. Accessed 28 Jan. 2019.
- U.S. Army. Army Disease Reporting System internet. Medical Event Report, 2018. Accessed 5 April 2018.
- 13. Ambrose J, Hampton LM, Fleming-Dutra KE, et al. Large outbreak of Legionnaires' disease and Pontiac fever at a military base. Epidemiol Infect. 2014;142(11):2336–2346.
- Dawood FS, Ambrose JF, Russell BP, et al. Outbreak of pneumonia in the setting of fatal pneumococcal meningitis among US Army trainees: potential role of Chlamydia pneumoniae infection. BMC Infect Dis. 2011;11:157.
- U.S Army. Army Disease Reporting System internet. Outbreak Report, 2019. Accessed 12 Feb. 2019.
- Army Public Health Center. Epidemiology Surveillance Training: Overview of Outbreak Methodology, 2018. http://phc.amedd.army.mil/topics/healthsurv/de/Pages/Epi-TechTraining.aspx. Accessed 12 Feb. 2019.
- Army Public Health Center. Epidemiology Surveillance Training: Developing a Standard Operating Procedure for Surveillance, 2019. http://phc.amedd.army.mil/topics/healthsurv/de/Pages/Epi-TechTraining.aspx. Accessed 12 Feb. 2019.